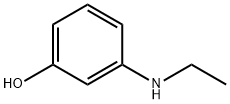
3-ETHYLAMINOPHENOL
- CAS No.
- 621-31-8
- Chemical Name:
- 3-ETHYLAMINOPHENOL
- Synonyms
- 3-(ethylamino)phenol;m-(ethylamino)phenol;m-(ethylamino)-pheno;3-(ethylamino)-pheno;Phenol, 3-(ethylamino)-;3-Hydroxyphenylethylamine
- CBNumber:
- CB5501890
- Molecular Formula:
- C8H11NO
- Molecular Weight:
- 137.18
- MOL File:
- 621-31-8.mol
- Modify Date:
- 2023/5/4 15:15:15
| Melting point | 62°C |
|---|---|
| Boiling point | 252.03°C (rough estimate) |
| Density | 1.0630 (rough estimate) |
| refractive index | 1.5560 (estimate) |
| storage temp. | 2-8°C |
| solubility | Chloroform (Slightly), Methanol (Slightly) |
| form | Solid |
| pka | 10.36±0.10(Predicted) |
| color | Light Brown to Very Dark Red |
| EPA Substance Registry System | 3-(Ethylamino)phenol (621-31-8) |
SAFETY
Risk and Safety Statements
| Symbol(GHS) |  GHS07 |
|---|---|
| Signal word | Warning |
| Hazard statements | H302-H412 |
| Precautionary statements | P264-P270-P301+P312-P330-P501-P273-P501 |
| Risk Statements | 34 |
| Safety Statements | 26-36/37/39 |
| RIDADR | 1759 |
| HS Code | 2922290090 |
3-ETHYLAMINOPHENOL Chemical Properties,Uses,Production
General Description
Very viscous deep orange liquid.
Air & Water Reactions
3-ETHYLAMINOPHENOL may be sensitive to prolonged exposure to air. . Slightly soluble in water.
Reactivity Profile
An amine and phenol. Amines are chemical bases. They neutralize acids to form salts plus water. These acid-base reactions are exothermic. The amount of heat that is evolved per mole of amine in a neutralization is largely independent of the strength of the amine as a base. Amines may be incompatible with isocyanates, halogenated organics, peroxides, phenols (acidic), epoxides, anhydrides, and acid halides. Flammable gaseous hydrogen is generated by amines in combination with strong reducing agents, such as hydrides. Phenols do not behave as organic alcohols, as one might guess from the presence of a hydroxyl (-OH) group in their structure. Instead, they react as weak organic acids. Phenols and cresols are much weaker as acids than common carboxylic acids (phenol has Ka = 1.3 x 10^[-10]). These materials are incompatible with strong reducing substances such as hydrides, nitrides, alkali metals, and sulfides. Flammable gas (H2) is often generated, and the heat of the reaction may ignite the gas. Heat is also generated by the acid-base reaction between phenols and bases. Such heating may initiate polymerization of the organic compound. Phenols are sulfonated very readily (for example, by concentrated sulfuric acid at room temperature). The reactions generate heat. Phenols are also nitrated very rapidly, even by dilute nitric acid.
Fire Hazard
Flash point data for 3-ETHYLAMINOPHENOL are not available, however, 3-ETHYLAMINOPHENOL is probably combustible.
3-ETHYLAMINOPHENOL Preparation Products And Raw materials
Raw materials
Preparation Products
| Supplier | Tel | Country | ProdList | Advantage | Inquiry |
|---|---|---|---|---|---|
| SUMUKHA LIFESCIENCES | +919000487488 | Hyderabad, India | 200 | 58 | Inquiry |
| A.J Chemicals | 91-9810153283 | New Delhi, India | 6124 | 58 | Inquiry |
| Shanghai Daken Advanced Materials Co.,Ltd | +86-371-66670886 | China | 16220 | 58 | Inquiry |
| SHANGHAI FDC-CHEMICAL CO.,LTD | 021-61469567 18521078705 | China | 20000 | 58 | Inquiry |
| Zhengzhou Edward Biology Co.,Ltd | 0371-63682289 15538176268 | China | 9995 | 58 | Inquiry |
| Shanghai Haohong Pharmaceutical Co., Ltd. | 4008210725 4008210725 | China | 55023 | 58 | Inquiry |
| Jinan Jiachang Pharmaceutical Technology Co. , Ltd. | 15063360100 | China | 147 | 58 | Inquiry |
| Shanghai Thunder Biological Technology Co., Ltd. | 18621609968 | China | 588 | 58 | Inquiry |
| Kangyue Huilan Pharmaceutical Technology Co., Ltd | 028-81092800 17323275954 | China | 511 | 58 | Inquiry |
| Henan Fangding Technology Co., LTD | 15670369782 15670369782 | China | 9838 | 58 | Inquiry |
621-31-8(3-ETHYLAMINOPHENOL)Related Search:
1of4
chevron_right




