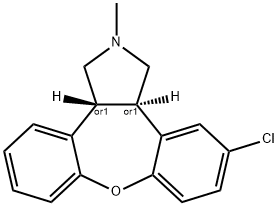
Asenapine
- CAS No.
- 65576-45-6
- Chemical Name:
- Asenapine
- Synonyms
- Saphris;HSDB8061;Asenapine;HSDB 8061;HSDB-8061;Unii-jkz19V908o;Einecs 265-829-4;Asenapine [inn:ban];Asenapine USP/EP/BP;Asenapine free base
- CBNumber:
- CB5879263
- Molecular Formula:
- C17H16ClNO
- Molecular Weight:
- 285.772
- MOL File:
- 65576-45-6.mol
- MSDS File:
- SDS
- Modify Date:
- 2024/7/2 8:55:12
SAFETY
Risk and Safety Statements
| Symbol(GHS) |  GHS07 |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Signal word | Warning | |||||||||
| Hazard statements | H319-H315-H335 | |||||||||
| Precautionary statements | P264-P280-P302+P352-P321-P332+P313-P362-P264-P280-P305+P351+P338-P337+P313P | |||||||||
| NFPA 704 |
|
Asenapine Chemical Properties,Uses,Production
Description
(±)-Asenapine is an atypical antipsychotic. It binds to dopamine D1-4, α-adrenergic, and histamine receptors (Kis = 0.42-1.45, 0.32-1.26, and 1-6.17 nM, respectively), as well as the serotonin (5-HT) receptor subtypes 5-HT1A, 5-HT1B, 5-HT2A, 5-HT2B, 5-HT2C, 5-HT5A, 5-HT6, and 5-HT7 (Kis = 0.03-3.98 nM). (±)-Asenapine inhibits the suppression of neuron firing induced by the 5-HT2A, dopamine D2, and α2-adrenergic receptor agonists 2,5-dimethoxy-4-iodoamphetamine (DOI), apomorphine, and clonidine , respectively, in rat brain (ED50s = 75, 40, and 85 μg/kg, respectively). In vivo, (±)-asenapine (0.05-0.2 mg/kg, s.c.) increases extracellular dopamine levels in the medial prefrontal cortex (mPFC), nucleus accumbens (NAc), and lateral striatum and suppresses the conditioned avoidance response in rats. It prevents acute and chronic phencyclidine-induced deficits in cued reversal learning in rats when administered at a dose of 0.075 mg/kg. Formulations containing asenapine have been used in the treatment of schizophrenia and bipolar I disorder.
Chemical Properties
Yellow Oil
Uses
Combined serotonin (5HT2) and dopamine (D2) receptor antagonist; structurally related to Mianserin. Antipsychotic
Biological Activity
Novel psychopharmacologic agent. Displays antagonist activity at 5-HT, dopamine, noradrenalin and histamine receptor subtypes (pK i values are 8.60, 8.40, 10.15, 9.75, 10.46, 8.84, 9.60, 9.94, 8.85, 8.90, 8.84, 9.38, 8.95, 8.93, 8.9, 9.49, 8.91, 9.00 and 8.21 for 5-HT 1A , 5-HT 1B , 5-HT 2A , 5-HT 2B , 5-HT 2C , 5-HT 5A , 5-HT 6 , 5-HT 7 , D 1 , D 2L , D 2S , D 3 , D 4 , α 1A , α 2A , α 2B , α 2C , H 1 and H 2 receptors respectively). Displays no appreciable affinity for muscarinic receptors. Exhibits potent activity in animal models predictive of antipsychotic efficacy.
Enzyme inhibitor
This atypical antipsychotic agent (FW = 285.77 g/mol; CAS 65576-45-6), marketed under the trade names Saphris ?, and also known as Org 5222 and (3aRS,12bRS)-rel-5-chloro-2,3,3a,12b-tetrahydro-2-methyl-1H-dibenz[2,3: 6,7]-oxepino-[4,5-c]pyrrole, is multi-receptor antagonist with the following spectrum of binding interactions: serotonin 5-HT1A receptor, Ki = 2.5 nM; serotonin 5-HT1B receptor, Ki = 4.0 nM; serotonin 5-HT2A receptor, Ki = 0.06 nM; serotonin 5-HT2B receptor, Ki = 0.16 nM; serotonin 5-HT2C receptor, Ki = 0.03 nM; serotonin 5-HT5A receptor, Ki = 1.6 nM; serotonin 5-HT6 receptor, Ki = 1.5 nM; serotonin 5-HT7 receptor, Ki = 0.13 nM; a1- Adrenergic receptor, Ki = 1.2 nM; a2A-Adrenergic receptor, Ki = 1.2 nM; a2B-Adrenergic receptor, Ki = 0.25 nM; a2C-Adrenergic receptor, Ki = 1.2 nM; dopamine D1-receptor, Ki = 1.4 nM; dopamine D2-receptor, Ki = 1.3 nM; dopamine D3-receptor, Ki = 0.4 nM; dopamine D4-receptor, Ki = 1.1 nM; histamine H1-receptor, Ki = 1.0 nM; and histamine H2-receptor, Ki = 6 nM. Like other atypical antipsychotic drugs, asenapine preferentially enhances dopamine and acetylcholine efflux in the rat medial prefrontal cortex and hippocampus. See Reference-x for asenapine’s UV, IR, NMR, and mass spectra as well as X-ray analysis, thermal properties, solubilities and partition coefficient.
Asenapine Preparation Products And Raw materials
Raw materials
Preparation Products
| Supplier | Tel | Country | ProdList | Advantage | Inquiry |
|---|---|---|---|---|---|
| VGS SYNTHESIS PRIVATE LIMITED | +91-9866975588 +91-9866975588 | Hyderabad, India | 1818 | 58 | Inquiry |
| Ebenezer Industries | Gujarat, India | 96 | 58 | Inquiry | |
| SynZeal Research Pvt Ltd | +1 226-802-2078 | Gujarat, India | 6522 | 58 | Inquiry |
| Pharmaffiliates Analytics and Synthetics P. Ltd | +91-172-5066494 | Haryana, India | 6773 | 58 | Inquiry |
| Chiral Biosciences Limited | 09441920381 | Mallapur, India | 41 | 58 | Inquiry |
| CLEARSYNTH LABS LTD. | +91-22-45045900 | Hyderabad, India | 6351 | 58 | Inquiry |
| Pharma Affiliates | 172-5066494 | Haryana, India | 6761 | 58 | Inquiry |
| ATK CHEMICAL COMPANY LIMITED | +undefined-21-51877795 | China | 32760 | 60 | Inquiry |
| career henan chemical co | +86-0371-86658258 +8613203830695 | China | 29898 | 58 | Inquiry |
| Chongqing Chemdad Co., Ltd | +86-023-6139-8061 +86-86-13650506873 | China | 39916 | 58 | Inquiry |
65576-45-6(Asenapine)Related Search:
1of4
chevron_right




