viomycin
- CAS No.
- 32988-50-4
- Chemical Name:
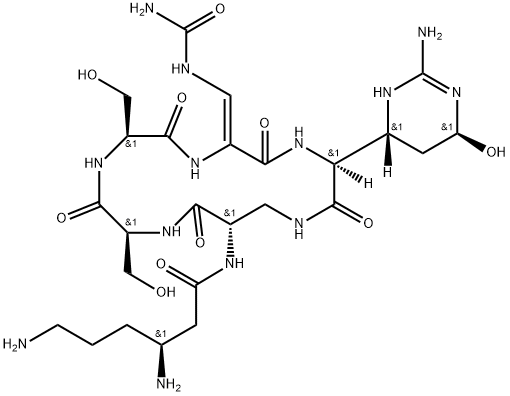
- viomycin
- Synonyms
- Viocin;viomycin;Vioactane;Coliomycin;FLORIMYCIN;Vinacetin A;Tuberactinomycin B;viomycin USP/EP/BP;Glycine, 3-amino-N-[(3S)-3,6-diamino-1-oxohexyl]-L-alanyl-L-seryl-L-seryl-(2Z)-3-[(aminocarbonyl)amino]-2,3-didehydroalanyl-2-[(4R,6S)-2-amino-1,4,5,6-tetrahydro-6-hydroxy-4-pyrimidinyl]-, (5→13)-lactam, (2S)-;(S)-3,6-Diamino-N-((3S,9S,12S,15S,Z)3((2R,4S)-6-amino-4-hydroxy-1,2,3,4-tetrahydropyridin-2-yl)-9,12-bis(hydroxymethyl)-2,5,8,11,14-pentaoxo-6-(ureidomethylene)-1,4,7,10,13-pentaazacyclohexadecan-15-yl)hexanamidedisulfate
- CBNumber:
- CB5910382
- Molecular Formula:
- C25H43N13O10
- Molecular Weight:
- 685.7
- MOL File:
- 32988-50-4.mol
- MSDS File:
- SDS
- Modify Date:
- 2024/3/16 16:41:06
| Melting point | 280 °C |
|---|---|
| Boiling point | 695.81°C (rough estimate) |
| Density | 1.3613 (rough estimate) |
| refractive index | 1.6700 (estimate) |
| storage temp. | Store at -20°C |
| solubility | Water (Slightly) |
| form | Powder |
| pka | pKa 8.2 (Uncertain);10.3 (Uncertain) |
| Water Solubility | Soluble to 75 mM in water |
| Stability | Hygroscopic |
SAFETY
Risk and Safety Statements
| Symbol(GHS) |  GHS07 |
|---|---|
| Signal word | Warning |
| Hazard statements | H302 |
| Precautionary statements | P264-P270-P301+P312-P330-P501 |
viomycin Chemical Properties,Uses,Production
Description
Viomycin was found independently in 1951, in the culture broth of Streptomyces puniceus by Pfizer Research Laboratories and in that of S. floridae by Parke Davis Co. It shows strong activity against Mycobacterium species and moderate activity against gram-positive and gram-negative bacteria. Viomycin has been used by intramuscular administration to treat tuberculosis, but because of its ototoxicity and renal toxicity it is being replaced by more active and less toxic drugs, such as enviomycin and other antituberculotic antibiotics.
Indications
Viomycin is a complex polypeptide antibiotic that is active against MDR strains of tuberculosis. Cross-resistance between viomycin and kanamycin is less frequent than between viomycin and capreomycin.
Definition
ChEBI: A cyclic peptide antibiotic produced by the actinomycete Streptomyces puniceus, used in the treatment of tuberculosis.
Biological Activity
Member of the tuberactinomycin family of antibiotics . Inhibits group I intron splicing and prokaryotic protein synthesis. Freezes bacterial ribosomes in either the pre- or post-translational state. Facilitates trans -cleavage of the Neurospora VS ribozyme.
viomycin Preparation Products And Raw materials
Raw materials
Preparation Products
viomycin Suppliers
| Supplier | Tel | Country | ProdList | Advantage | Inquiry |
|---|---|---|---|---|---|
| TargetMol Chemicals Inc. | +1-781-999-5354 +1-00000000000 | United States | 19892 | 58 | Inquiry |
| Shaanxi Dideu Medichem Co. Ltd | +86-029-89586680 +86-18192503167 | China | 9030 | 58 | Inquiry |
| BOC Sciences | 16314854226; +16314854226 | United States | 19743 | 58 | Inquiry |
| Shaanxi Cuikang Pharmaceutical Technology Co., Ltd | +86-19164747840 +86-13119157289 | China | 2969 | 58 | Inquiry |
| United States Biological | 800.520.3011 or 781.639.5092 | United States | 6256 | 80 | Inquiry |
| MedBioPharmaceutical Technology Inc | 021-69568360 18916172912 | China | 8141 | 58 | Inquiry |
| Shaanxi Dideu Newmaterial Co., Ltd. | 029-63373950 15353716720 | China | 10011 | 58 | Inquiry |
| Lanospharma Laboratories Co.,Ltd | 13440048448 | China | 6343 | 56 | Inquiry |
| 3B Pharmachem (Wuhan) International Co.,Ltd. | 821-50328103-801 18930552037 | China | 15848 | 69 | Inquiry |
| Shanghai New Union Textra Import & Export Co., Ltd | +861-348-227-9455 | China | 2752 | 60 | Inquiry |
32988-50-4(viomycin)Related Search:
1of2
chevron_right




