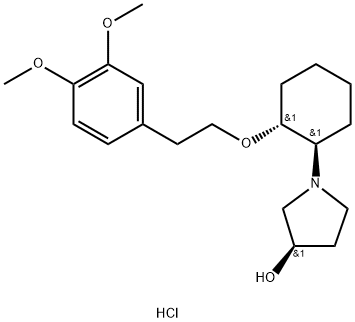
VERNAKALANT HYDROCHLORIDE
- CAS No.
- 748810-28-8
- Chemical Name:
- VERNAKALANT HYDROCHLORIDE
- Synonyms
- D06665;Vernakalant HCl;RSD1235 hydrochloride;RSD 1235 hydrochloride;RSD-1235 hydrochloride;Mepivacaine Impurity 8;VERNAKALANT HYDROCHLORIDE;Vernakalant hydrochloride (usan);Vernakalant ((3R,1'R,2'R)-Isomer) HCl;Vernakalant ((3R,1'R,2'R)-Isomer) HCl
- CBNumber:
- CB61011678
- Molecular Formula:
- C20H32ClNO4
- Molecular Weight:
- 385.93
- MOL File:
- 748810-28-8.mol
- MSDS File:
- SDS
- Modify Date:
- 2023/5/18 11:31:06
SAFETY
Risk and Safety Statements
| Symbol(GHS) |  GHS07 |
|---|---|
| Signal word | Warning |
| Hazard statements | H332-H302-H335-H319-H312-H315 |
| Precautionary statements | P264-P270-P301+P312-P330-P501-P280-P302+P352-P312-P322-P363-P501-P261-P271-P304+P340-P312-P264-P280-P305+P351+P338-P337+P313P-P264-P280-P302+P352-P321-P332+P313-P362 |
VERNAKALANT HYDROCHLORIDE Chemical Properties,Uses,Production
Uses
Vernakalant Hydrochloride is a novel, relatively atrial-selective antiarrhythmic drug that effectively and rapidly terminates atrial fibrillation (AF).
Clinical Use
Vernakalant is an investigational drug under regulatory review for the acute conversion of atrial fibrillation. The drug was initially developed by Cardiome Pharma under the trade names Kynapid ® and Brinavess ® and its intravenous formulation was further developed by Merck in April 2009. Like other class III antiarrhythmics, vernakalant blocks atrial potassium channels, thereby prolonging repolarization. It differs from typical class III agents by blocking the cardiac transient outward potassium current, with increased potency as the heart rate increases. It also slightly blocks the hERG potassium channel, leading to a prolonged QT interval, which may theoretically increase the risk of ventricular tachycardia.
Synthesis
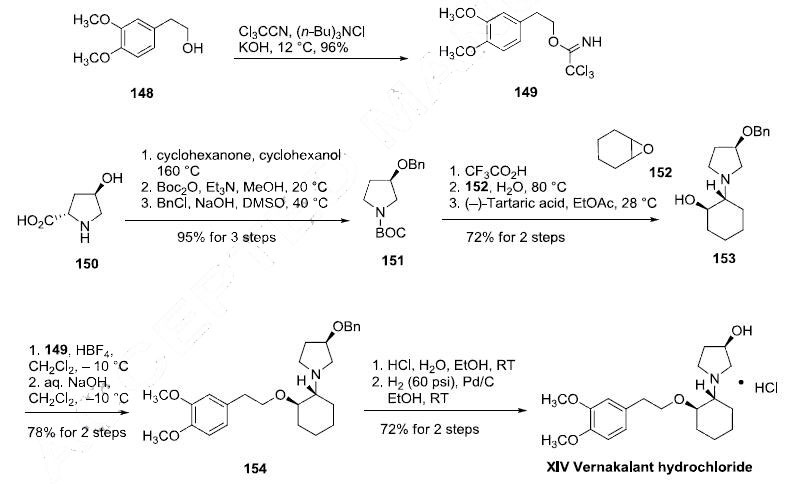
The preparation of vernakalant entails the union of a prolinol derivative 150 with a 3,4-dimethoxyphenethyl alcohol (148) across a cyclohexanyl lynchpin 152 and is described in the scheme. Decarboxylation of commercially available (2S,4R)-4- hydroxyprolinol (150) was effected using cyclohexanol and cyclohexanone at elevated temperatures. Subsequent protection of the nitrogen atom and the oxygen atom within this system resulted in carbamate 151. Acid-mediated removal of the N-protective functionality preceded nucleophilic attack on epoxide 152 in hot water, and the ensuing mixture of diastereomers was separated by classical resolution via the tartrate salt. O-Benzylated vernakalant 154 was obtained when enantiomerically pure alcohol 153 was subjected to trichloroacetimidate 149 (which arose from the corresponding alcohol 148 under modified Williamson conditions. Acidic hydrogenolysis, which the authors report as separate steps, furnished vernakalant hydrochloride (XIV) in excellent overall yield.
VERNAKALANT HYDROCHLORIDE Preparation Products And Raw materials
Raw materials
Preparation Products
| Supplier | Tel | Country | ProdList | Advantage | Inquiry |
|---|---|---|---|---|---|
| CLEARSYNTH LABS LTD. | +91-22-45045900 | Hyderabad, India | 6351 | 58 | Inquiry |
| A.J Chemicals | 91-9810153283 | New Delhi, India | 6124 | 58 | Inquiry |
| Henan Tianfu Chemical Co.,Ltd. | +86-0371-55170693 +86-19937530512 | China | 21669 | 55 | Inquiry |
| CONIER CHEM AND PHARMA LIMITED | +8618523575427 | China | 49391 | 58 | Inquiry |
| TargetMol Chemicals Inc. | +1-781-999-5354 +1-00000000000 | United States | 19892 | 58 | Inquiry |
| Zhejiang J&C Biological Technology Co.,Limited | +1-2135480471 +1-2135480471 | China | 10522 | 58 | Inquiry |
| Aladdin Scientific | +1-+1(833)-552-7181 | United States | 57511 | 58 | Inquiry |
| Amadis Chemical Company Limited | 571-89925085 | China | 131980 | 58 | Inquiry |
| AdooQ Bioscience CHINA | 025-58849295 18951903616; | China | 2989 | 60 | Inquiry |
| Shanghai Weiwei Biological Technology Co., Ltd. | 021-55669583 18616258598 | CHINA | 4398 | 58 | Inquiry |





