Hypophosphorous acid
- CAS No.
- 6303-21-5
- Chemical Name:
- Hypophosphorous acid
- Synonyms
- PHOSPHINIC ACID;HYPOPHOSPHORUS ACID;HYPOPHOSPHORIC ACID;hypophosphorous;Hypophoaphoeous acid;Hypophosphorous acid 50%;Hypophosphorousacid,50%w/waq.soln.;Dihydroxyphosphine;Hypophosphite Acid;phosphinic acid 50%
- CBNumber:
- CB6130318
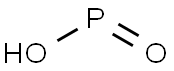
- Molecular Formula:
- HO2P
- Molecular Weight:
- 63.980501
- MOL File:
- 6303-21-5.mol
- MSDS File:
- SDS
- Modify Date:
- 2024/6/11 19:18:13
| Melting point | -25 °C |
|---|---|
| Boiling point | 108 °C (759.8513 mmHg) |
| Density | 1.206 g/mL at 20 °C(lit.) |
| vapor pressure | <17 mmHg ( 20 °C) |
| storage temp. | no restrictions. |
| solubility | very soluble in H2O, ethanol, ethyl ether |
| pka | pK1 1.1. |
| form | hygroscopic crystals or colorless oily liquid |
| color | Colorless |
| Water Solubility | SOLUBLE |
| Merck | 13,4894 |
| Stability | Stable. Incompatible with strong bases. Reacts violently with oxidizing agents, strong bases, mercury (II) nitrate and mercury (II) oxide. Do not heat above 100 C. |
| InChIKey | GQZXNSPRSGFJLY-UHFFFAOYSA-N |
| CAS DataBase Reference | 6303-21-5(CAS DataBase Reference) |
| NIST Chemistry Reference | Hypophosphorous acid(6303-21-5) |
| EPA Substance Registry System | Phosphinic acid (6303-21-5) |
SAFETY
Risk and Safety Statements
| Symbol(GHS) |  GHS05 |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Signal word | Danger | |||||||||
| Hazard statements | H290-H314 | |||||||||
| Precautionary statements | P234-P280-P301+P330+P331-P303+P361+P353-P304+P340+P310-P305+P351+P338 | |||||||||
| Hazard Codes | C | |||||||||
| Risk Statements | 34 | |||||||||
| Safety Statements | 26-36/37/39-45 | |||||||||
| RIDADR | UN 3264 8/PG 3 | |||||||||
| WGK Germany | 1 | |||||||||
| RTECS | SZ6400000 | |||||||||
| TSCA | Yes | |||||||||
| HazardClass | 8 | |||||||||
| PackingGroup | II | |||||||||
| HS Code | 28111990 | |||||||||
| NFPA 704 |
|
Hypophosphorous acid price More Price(12)
| Manufacturer | Product number | Product description | CAS number | Packaging | Price | Updated | Buy |
|---|---|---|---|---|---|---|---|
| Sigma-Aldrich(India) | 214906 | Hypophosphorous acid solution 50?wt. % in H2O | 6303-21-5 | 100ML | ₹2576.35 | 2022-06-14 | Buy |
| Sigma-Aldrich(India) | 214906 | Hypophosphorous acid solution 50?wt. % in H2O | 6303-21-5 | 500ML | ₹9461.05 | 2022-06-14 | Buy |
| Sigma-Aldrich(India) | 1.04633 | Hypophosphorous acid 50% for analysis EMSURE? | 6303-21-5 | 500ML | ₹28360 | 2022-06-14 | Buy |
| ALFA India | ALF-014142-57 | Hypophosphorous Acid, 50% w/w Aqueous Solution | 6303-21-5 | 4x500g | ₹9423 | 2022-05-26 | Buy |
| ALFA India | ALF-014142-36 | Hypophosphorous acid, 50% w/w aq. soln. | 6303-21-5 | 500g | ₹7730 | 2022-05-26 | Buy |
Hypophosphorous acid Chemical Properties,Uses,Production
Description
Hypophosphorous acid is a powerful reducing agent with a molecular formula of H3PO2. Inorganic chemists refer to the free acid by this name although its IUPAC name is dihydridohydroxidooxidophosphorus, or the acceptable name of phosphinic acid. It is a colorless low-melting compound, which is soluble in water, dioxane, and alcohols. The formula for hypophosphorous acid is generally written H3PO2, but a more descriptive presentation is HOP(O)H2 which highlights its monoprotic character. Salts derived from this acid are called phosphinates (hypophosphites).
Chemical Properties
colourless liquid
Physical properties
Colorless deliquescent crystals or oily liquid; sour odor; density 1.493 g/cm3;melts at 26.5°C; boils at 130°C; very soluble in water, alcohol and ether; den-sity of a 50% aqueous solution is 1.13 g/mL.
Uses
Hypophosphorous acid is primarily used for electroless nickel plating. It is involved in the reduction of arenediazonium salts. It acts as an additive in Fischer esterification reactions. Also, it serves as a neutralizing agent, antioxidant, catalyst in polymerization and poly condensation, and wetting agent. Further, it is used in the formulation of pharmaceuticals, discoloration of polymers, water treatment and retrieval of precious or non-ferrous metals. In addition to this, it is used as bleaching agents for plastics, synthetic fibers, decolorizing agent and for color stabilization during the manufacture of chemicals and several plastics.
Production Methods
Hypophosphorous acid is formed by reaction of barium hypophosphite and sulfuric acid, and filtering off barium sulfate. By evaporation of the solution in vacuum at 80 °C, and then cooling to 0°C, hypophosphorous acid crystallizes.
Definition
ChEBI: A phosphorus oxoacid that consists of a single pentavalent phosphorus covalently bound via single bonds to two hydrogens and a hydroxy group and via a double bond to an oxygen. The parent of the class of phosphinic acids.
Preparation
Hypophosphorous acid may be prepared by various methods:
1. Boiling white phosphorus with calcium hydroxide:
P4 + 4Ca(OH)2 + 8H2O → 4Ca(H2PO2)2 + 4H2
The calcium salt is soluble in water. Treatment with sulfuric acid yields thehypophosphorous acid:
(H2PO2)2Ca + H2SO4 → 2H3PO2 + CaSO4
The product mixture is filtered to remove insoluble CaSO4. The aqueous solu-tion of hypophosphorous acid is concentrated under reduced pressure.Concentrated baryta water may be used instead of calcium hydroxide.2. By treating sodium hypophosphite, NaH2PO2with an ion-exchange resin.The sodium salt may be produced by boiling white phosphorus with a solutionof sodium hydroxide, a reaction similar to (1) above.
PH3 + 2I2 + 2H2O → H3PO2 + 4HI
The above method may be considered safer than that involving heating whitephosphorus with an alkali.
Hypophosphorous acid must be stored below 50°C. It is sold commerciallyas an aqueous solution at various concentrations.
Reactions
Hypophosphorous acid is miscible with water in all proportions and a commercial strength is 30% H3PO2. Hypophosphites are used in medicine. Hypophosphorous acid is a powerful reducing agent, e.g., with copper sulfate forms cuprous hydride Cu2H2, brown precipitate, which evolves hydrogen gas and leaves copper on warming; with silver nitrate yields finely divided silver; with sulfurous acid yields sulfur and some hydrogen sulfide; with sulfuric acid yields sulfurous acid, which reacts as above; forms manganous immediately with permanganate.
General Description
Hypophosphorous acid appears as colorless oily liquid or deliquescent crystals with a sour odor. Density 1.439 g / cm3. Melting point 26.5°C. Inhalation of vapors irritates or burns the respiratory tract. Liquid and vapors may irritate or burn eyes and skin.
Air & Water Reactions
Deliquescent. Water soluble.
Reactivity Profile
HYPOPHOSPHOROUS ACID decomposes when heated into phosphoric acid and spontaneously flammable phosphine. Is oxidized by sulfuric acid with release of sulfur dioxide and sulfur. Reacts explosively with mercury(II) oxide [Mellor, 1940, Vol. 4, 778]. Reacts violently with mercury(II) nitrate [Mellor, 1940, Vol. 4, 993]. Neutralizes bases in exothermic reactions.
Hazard
Fire and explosion risk in contact with oxidizing agents.
Health Hazard
TOXIC; inhalation, ingestion or skin contact with material may cause severe injury or death. Contact with molten substance may cause severe burns to skin and eyes. Avoid any skin contact. Effects of contact or inhalation may be delayed. Fire may produce irritating, corrosive and/or toxic gases. Runoff from fire control or dilution water may be corrosive and/or toxic and cause pollution.
Fire Hazard
Non-combustible, substance itself does not burn but may decompose upon heating to produce corrosive and/or toxic fumes. Some are oxidizers and may ignite combustibles (wood, paper, oil, clothing, etc.). Contact with metals may evolve flammable hydrogen gas. Containers may explode when heated.
Purification Methods
Phosphorous acid is a common contaminant of commercial 50% hypophosphorous acid. Jenkins and Jones [J Am Chem Soc 74 1353 1952] purified this material by evaporating about 600mL in a 1L flask at 40o, under reduced pressure (in N2), to a volume of about 300mL. After the solution was cooled, it was transferred to a wide-mouthed Erlenmeyer flask which was stoppered and left in a Dry-ice/acetone bath for several hours to freeze (if necessary, with scratching of the wall). When the flask was then left at ca 5o for 12hours, about 30-40% of it liquefied, and was again filtered. This process was repeated, then the solid was stored over Mg(ClO4)2 in a vacuum desiccator in the cold. Subsequent crystallisations from n-butanol by dissolving it at room temperature and then cooling in an ice-salt bath at -20o did not appear to purify it further. The free acid forms deliquescent crystals m 26.5o and is soluble in H2O and EtOH. The NaH2PO2 salt can be purified through an anion exchange resin [Klement Z Anorg Allgem Chem 260 267 1949.]
Hypophosphorous acid Preparation Products And Raw materials
Raw materials
Preparation Products
1of2
chevron_right| Supplier | Tel | Country | ProdList | Advantage | Inquiry |
|---|---|---|---|---|---|
| JSK Chemicals | +919879767970 | Gujarat, India | 3756 | 58 | Inquiry |
| Anish Chemicals | +919429005558 | Gujarat, India | 13 | 58 | Inquiry |
| Neemcco | +91-2222051070 +91-9820039043 | Maharashtra, India | 4 | 58 | Inquiry |
| Anubhav Business Corporation | +91-2228014519 +91-2228014999 | Maharashtra, India | 119 | 58 | Inquiry |
| Prasol Chemicals Pvt Ltd | 91-22-27782555 | Maharashtra, India | 27 | 58 | Inquiry |
| Victory Chemicals | +91-2224175189 +91-9820098499 | Maharashtra, India | 9 | 58 | Inquiry |
| S D Fine Chem Limited | +91-9323715856 +91-9323715856 | Maharashtra, India | 368 | 58 | Inquiry |
| Anan Drug Chem Ltd. | +91-2782540604 +91-2782540604 | Gujarat, India | 5 | 58 | Inquiry |
| Punjab Chemicals & Crop Protection Limited | +91 (22) 2674-7900 | New Delhi, India | 125 | 0 | Inquiry |
| Scientific OEM | +91-22- 2343 7546 / 2341 3094 | New Delhi, India | 1996 | 38 | Inquiry |
| Supplier | Advantage |
|---|---|
| JSK Chemicals | 58 |
| Anish Chemicals | 58 |
| Neemcco | 58 |
| Anubhav Business Corporation | 58 |
| Prasol Chemicals Pvt Ltd | 58 |
| Victory Chemicals | 58 |
| S D Fine Chem Limited | 58 |
| Anan Drug Chem Ltd. | 58 |
| Punjab Chemicals & Crop Protection Limited | 0 |
| Scientific OEM | 38 |
Related articles
- Preparation of Hypophosphorous acid
- Hypophosphorous acid is the starting product for the synthesis of organophosphorus compounds.
- Jun 20,2022
6303-21-5(Hypophosphorous acid)Related Search:
1of4
chevron_right




