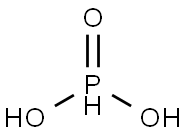
Phosphorous acid
- CAS No.
- 13598-36-2
- Chemical Name:
- Phosphorous acid
- Synonyms
- PHOSPHONIC ACID;PHOSPHORUS ACID;Phosphorous acid 99%;Phosphonsure;Phospohorous acid;Phosphorous Acid Crystal;AURORA KA-1076;orthophosphorus;ORTHOPHOSPHOROUS;Phosphorous acid
- CBNumber:
- CB6700409
- Molecular Formula:
- H3O3P
- Molecular Weight:
- 82
- MOL File:
- 13598-36-2.mol
- MSDS File:
- SDS
- Modify Date:
- 2024/1/8 13:19:30
| Melting point | 73 °C |
|---|---|
| Boiling point | 200 °C |
| Density | 1.651 g/mL at 25 °C(lit.) |
| vapor pressure | 0.001Pa at 20℃ |
| Flash point | 200°C |
| storage temp. | 0-6°C |
| solubility | DMSO (Slightly), Methanol (Slightly), Water (Sparingly) |
| form | Crystals |
| pka | pK1 1.29; pK2 6.74(at 25℃) |
| Specific Gravity | 1.651 |
| color | White |
| Water Solubility | SOLUBLE |
| Sensitive | Air Sensitive & Hygroscopic |
| Merck | 14,7346 |
| Stability | Stable. Incompatible with strong bases. Hygroscopic. |
| CAS DataBase Reference | 13598-36-2(CAS DataBase Reference) |
| NIST Chemistry Reference | (HO)2HPO(13598-36-2) |
| EPA Substance Registry System | Phosphonic acid (13598-36-2) |
SAFETY
Risk and Safety Statements
| Symbol(GHS) |   GHS05,GHS07 |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Signal word | Danger | |||||||||
| Hazard statements | H290-H302-H314 | |||||||||
| Precautionary statements | P234-P270-P280-P301+P312-P303+P361+P353-P305+P351+P338 | |||||||||
| Hazard Codes | C | |||||||||
| Risk Statements | 22-35 | |||||||||
| Safety Statements | 26-36/37/39-45 | |||||||||
| RIDADR | UN 2834 8/PG 3 | |||||||||
| WGK Germany | 1 | |||||||||
| RTECS | SZ6400000 | |||||||||
| F | 3 | |||||||||
| TSCA | Yes | |||||||||
| HazardClass | 8 | |||||||||
| PackingGroup | III | |||||||||
| HS Code | 28092019 | |||||||||
| NFPA 704 |
|
Phosphorous acid price More Price(13)
| Manufacturer | Product number | Product description | CAS number | Packaging | Price | Updated | Buy |
|---|---|---|---|---|---|---|---|
| Sigma-Aldrich(India) | 215112 | Phosphorous acid 99% | 13598-36-2 | 100G | ₹1980.98 | 2022-06-14 | Buy |
| Sigma-Aldrich(India) | 215112 | Phosphorous acid 99% | 13598-36-2 | 500G | ₹3366.58 | 2022-06-14 | Buy |
| Sigma-Aldrich(India) | 215112 | Phosphorous acid 99% | 13598-36-2 | 2KG | ₹6419.23 | 2022-06-14 | Buy |
| Sigma-Aldrich(India) | 04115 | Phosphorous acid solution ≥50% | 13598-36-2 | 1L | ₹6776.45 | 2022-06-14 | Buy |
| ALFA India | ALF-A11189-30 | Phosphorous acid, 98+% | 13598-36-2 | 250g | ₹3325 | 2022-05-26 | Buy |
Phosphorous acid Chemical Properties,Uses,Production
Description
Phosphorous acid, H3PO3, is diprotic (readily ionizes two protons), not triprotic as might be suggested by this formula. Phosphorous acid is as an intermediate in the preparation of other phosphorous compounds. Because preparation and uses of “phosphorous acid” actually pertain more to the major tautomer, phosphonic acid, it is more often referred to as “phosphorous acid”. Phosphorous acid has the chemical formula H3PO3, which is best expressed as HPO(OH)2 to show its diprotic character. P(OH)3 (IUPAC: phosphorous acid) has CAS number 10294-56-1. It has been shown to be a stable tautomer.
Chemical Properties
Phosphorous acid is a white crystalline deliquescent solid that can be prepared by the action of water on phosphorus( III) oxide or phosphorus(III) chloride. It is a dibasic acid producing the anions H2PO3- and HPO3 2- in water. The acid and its salts are slow reducing agents. On warming, phosphonic acid decomposes to phosphine and phosphoric(V) acid. Phosphorus acid is used to prepare phosphite salts. It is usually sold as a 20% aqueous solution.
Physical properties
White crystalline mass; deliquescent; garlic-like odor; density 1.651 g/cm3 at 21°C; melts at 73.6°C; decomposes at 200°C to phosphine and phosphoric acid; soluble in water, about 310 g/100mL; K1 5.1x10-2 and K2 1.8x10-7; soluble in alcohol.
Uses
Phosphorous acid is used to produce the fertilizer phosphate salt like potassium phosphite, ammonium phosphite and calcium phosphite. It is actively involved in the preparation of phosphites like aminotris(methylenephosphonic acid) (ATMP), 1-hydroxyethane 1,1-diphosphonic acid (HEDP) and 2-phosphonobutane-1,2,4-tricarboxylic Acid (PBTC), which find application in water treatment as a scale or corrosive inhibitor. It is also used in chemical reactions as a reducing agent. Its salt, lead phosphite is used as PVC stabilizer. It is also used as a precursor in the preparation of phosphine and as an intermediate in the preparation of other phosphorus compounds.
Preparation
Phosphorus acid can be prepared by the reaction of phosphorus trichloride with water: PCl3 + 3H2O → H3PO4 + 3HClThe reaction is violent. Addition of PCl3 should be extremely cautious and slow. The addition can be carried out safely in the presence of concentrated HCl. Alternatively, a stream of air containing PCl3 vapor is passed into icecold water and solid crystals of H3PO4 form. Alternatively, phosphorus acid can be prepared by adding phosphorus trichloride to anhydrous oxalic acid: PCl3 + 3(COOH)2 → H3PO3 + 3CO + 3CO2 + 3HCl In this reaction, all products except H3PO3 escape as gases leaving the liquid acid. Dissolution of phosphorus sesquioxide in water also forms phosphorus acid. When shaken with ice water, phosphorus acid is the only product . P4O6 + 6H2O → 4H3PO3 However, in hot water part of the phosphorus acid disproportionates to phosphoric acid and phosphorus or phosphine.
Definition
ChEBI: Phosphorous acid is a phosphorus oxoacid. It is a conjugate acid of a dihydrogenphosphite. It is a tautomer of a phosphonic acid.
Application
Phosphorous acid (H3PO3, orthophosphorous acid) may be used as one of the reaction components for the synthesis of the following:
α-aminomethylphosphonic acids via Mannich-Type Multicomponent Reaction
1-aminoalkanephosphonic acids via amidoalkylation followed by hydrolysis
N-protected α-aminophosphonic acids (phospho-isosteres of natural amino acids) via amidoalkylation reaction
General Description
Phosphorous acid appears as a white or yellow crystalline solid (melting point 70.1 deg C) or a solution of the solid. Density 1.651 g /cm3 . Contact may severely irritate skin, eyes, and mucous membranes. Toxic by ingestion, inhalation and skin absorption.
Air & Water Reactions
Deliquescent. Absorbs oxygen from the air very readily to form phosphoric acid [Hawley]. Soluble in water.
Reactivity Profile
Phosphorous acid decomposes when heated to form phosphine, a gas that usually ignites spontaneously in air. Absorbs oxygen from the air to form phosphoric acid [Hawley]. Forms yellow deposits in aqueous solution that are spontaneously flammable upon drying. Reacts exothermically with chemical bases (for example: amines and inorganic hydroxides) to form salts. These reactions can generate dangerously large amounts of heat in small spaces. Dissolution in water or dilution of a concentrated solution with additional water may generate significant heat. Reacts in the presence of moisture with active metals, including such structural metals as aluminum and iron, to release hydrogen, a flammable gas. Can initiate the polymerization of certain alkenes. Reacts with cyanide compounds to release gaseous hydrogen cyanide. May generate flammable and/or toxic gases in contact with dithiocarbamates, isocyanates, mercaptans, nitrides, nitriles, sulfides, and strong reducing agents. Additional gas-generating reactions occur with sulfites, nitrites, thiosulfates (to give H2S and SO3), dithionites (to give SO2), and carbonates (to give CO2).
Health Hazard
TOXIC; inhalation, ingestion or skin contact with material may cause severe injury or death. Contact with molten substance may cause severe burns to skin and eyes. Avoid any skin contact. Effects of contact or inhalation may be delayed. Fire may produce irritating, corrosive and/or toxic gases. Runoff from fire control or dilution water may be corrosive and/or toxic and cause pollution.
Fire Hazard
Non-combustible, substance itself does not burn but may decompose upon heating to produce corrosive and/or toxic fumes. Some are oxidizers and may ignite combustibles (wood, paper, oil, clothing, etc.). Contact with metals may evolve flammable hydrogen gas. Containers may explode when heated.
Industrial uses
This collector was developed recently and was used primarily as specific collector for cassiterite from ores with complex gangue composition.On the basis of the phosphonic acid, Albright and Wilson had developed a range of collectors mainly for flotation of oxidic minerals (i.e. cassiterite, ilmenite and pyrochlore). Very little is known about the performance of these collectors. Limited studies conducted with cassiterite and rutile ores showed that some of these collectors produce voluminous froth but were very selective.
Safety Profile
Moderately toxic by ingestion. When heated to decomposition at 200℃ it emits toxic fumes of POx and phosphme whch may ignite. See also PHOSPHINE.
Phosphorous acid Preparation Products And Raw materials
Raw materials
Preparation Products
1of2
chevron_right| Supplier | Tel | Country | ProdList | Advantage | Inquiry |
|---|---|---|---|---|---|
| ANJI BIOSCIENCES | +91-9000100077 +91-9000100077 | Hyderabad, India | 430 | 58 | Inquiry |
| JSK Chemicals | +919879767970 | Gujarat, India | 3756 | 58 | Inquiry |
| PUNJAB CHEMICALS AND CROP PROTECTION LTD | +91-7508355205 +91-7508355205 | Punjab, India | 69 | 58 | Inquiry |
| Sandhya Group | +91-9167722041 +91-9167722043 | Maharashtra, India | 22 | 58 | Inquiry |
| Prasol Chemicals Pvt Ltd | 91-22-27782555 | Maharashtra, India | 27 | 58 | Inquiry |
| Otto Chemie Pvt. Ltd. | +91 9820041841 | Mumbai, India | 5873 | 58 | Inquiry |
| Alfa Aesar | 1 800 209 7001 | Maharashtra, India | 6913 | 58 | Inquiry |
| Sisco Research Laboratories Pvt. Ltd. | +91-22-4268 5800 | Mumbai, India | 4317 | 58 | Inquiry |
| Central Drug House(P) Ltd. | 91-11-49404040 | New Delhi, India | 6160 | 58 | Inquiry |
| Triveni chemicals | 08048762458 | New Delhi, India | 6093 | 58 | Inquiry |
| Supplier | Advantage |
|---|---|
| ANJI BIOSCIENCES | 58 |
| JSK Chemicals | 58 |
| PUNJAB CHEMICALS AND CROP PROTECTION LTD | 58 |
| Sandhya Group | 58 |
| Prasol Chemicals Pvt Ltd | 58 |
| Otto Chemie Pvt. Ltd. | 58 |
| Alfa Aesar | 58 |
| Sisco Research Laboratories Pvt. Ltd. | 58 |
| Central Drug House(P) Ltd. | 58 |
| Triveni chemicals | 58 |
Related articles
- Preparation and Application Phosphorous acid
- Phosphorous acid is mainly used in prostatic hypertrophy caused by urination disorders. It is a highly selective α1A receptor ....
- Nov 11,2022
13598-36-2(Phosphorous acid)Related Search:
1of4
chevron_right




