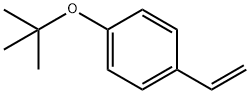
4-tert-Butoxystyrene
- CAS No.
- 95418-58-9
- Chemical Name:
- 4-tert-Butoxystyrene
- Synonyms
- P-T-BUTOXYSTYRENE;4-T-BUTOXYSTYRENE;p-tert-butoxystyrene;1-ethenyl;butoxy styrene;4-tert-butoxystyene;4-TERT-BUTOXYSTYRENE;(1-Butoxyvinyl)benzene;4-tert-Butyloxystyrene;p-(tert-Butyloxy)styrene
- CBNumber:
- CB6229094
- Molecular Formula:
- C12H16O
- Molecular Weight:
- 176.25
- MOL File:
- 95418-58-9.mol
- MSDS File:
- SDS
- Modify Date:
- 2024/6/4 16:27:49
| Melting point | −38 °C(lit.) |
|---|---|
| Boiling point | 256.8±9.0 °C Press: 760 Torr |
| Density | 0.936 g/mL at 25 °C(lit.) |
| vapor pressure | 17.999Pa at 20℃ |
| refractive index |
n |
| Flash point | 207 °F |
| InChI | InChI=1S/C12H16O/c1-5-10-6-8-11(9-7-10)13-12(2,3)4/h5-9H,1H2,2-4H3 |
| InChIKey | GRFNSWBVXHLTCI-UHFFFAOYSA-N |
| SMILES | C1(OC(C)(C)C)=CC=C(C=C)C=C1 |
| LogP | 3.83 at 22℃ and pH7 |
| CAS DataBase Reference | 95418-58-9(CAS DataBase Reference) |
SAFETY
Risk and Safety Statements
| Symbol(GHS) |  GHS07 |
|---|---|
| Signal word | Warning |
| Hazard statements | H315-H319-H335 |
| Precautionary statements | P261-P264-P271-P280-P302+P352-P305+P351+P338 |
| Hazard Codes | Xi |
| Risk Statements | 36/37/38 |
| Safety Statements | 26-36 |
| WGK Germany | 3 |
| HS Code | 2902900000 |
4-tert-Butoxystyrene Chemical Properties,Uses,Production
Description
4-tert-butoxystyrene is a pendant functionalized styrene that structurally, It is similar to PMOS, both carrying an alkoxyl p-substituent, whose electron-donating and resonance effects allow them to be'polymerized by cationic, anionic, and radical mechanisms. It is used to treatment of its polymers with an acid leads to poly(4-vinylphenol), which finds a wide variety of applications to photoresists, epoxy-curing agents, adhesives, etc.).
Chemical Properties
Colorless to Almost colorless clear liquid.
Uses
4-tert-Butoxystyrene is used in organic synthesis. It is used to synthesize 4'-tert-butoxy-biphenyl-4-carboxylic acid methyl ester.
Application
4-tert-Butoxystyrene is an organic synthetic reagent used as a polymer monomer in the preparation of poly(4-tert-butoxystyrene) (PtBSt) and other living polymers. Poly(4-tert-Butoxystyrene) is a new hexane-soluble polymer surfactant that can be used to form micelles.
Synthesis
Preparation of 4-tert-Butoxystyrene (3-tert-Butoxystyrene). To a 2000-mL three-necked round-bottom flask equipped with a dropping funnel, thermometer, reflux condenser, paddle stirrer and nitrogen inlet were placed 19.4 g (0.80 mol) of magnesium turnings and enough freshly dried and distilled tetrahydrofuran (THF) to cover the turnings. There were then added dropwise with stirring a solution of 143.3g (0.78 mol) of freshly distilled 4-bromostyrene (bp 46-47°C (0.03 mm)) in 500mL of THF. After 20mL of the 4-bromostyrene had been added, an exothermic reaction set in and was maintained between 25 and 35°C by adjusting the rate of addition of the bromo compound and with the aid of an ice bath. After the addition had been completed, the reaction mixture was heated to 60°C for 0.5h. Using a NaC1-ice bath, the mixture was cooled to 0°C and a solution of 100.88g (0.52 mol) of tert-butyl peroxybenzoate in 200 mL of THF was added via the dropping funnel at such a rate that the reaction temperature was maintained between 0 and 5°C. After completion of the addition, the reaction mixture was stirred at 25°C for 2 h. The organic layer was separated from the solid magnesium benzoate by decantation and the volume of the solution reduced on a rotary evaporator. The yellow oil that remained was washed with 1000 mL of 3% aqueous HCl solution and the organic layer separated. The aqueous layer was washed with two 200-mL portions of ether, and the ether and organic layers were combined and together washed with two 75-mL portions of a 10% NaOH solution followed by washing with water until the aqueous washings were neutral. After the solution was dried over Na2S04 and the ether was removed via a rotary evaporator, the remaining pale yellow oil was purified by fractional distillation in the presence of a few milligrams of ionol (2,6-ditert-butyl-4-methylphenol) as an inhibitor. There were obtained 46 g (50% yield, bp 45°C (0.02 mm)) of 4-tert-Butoxystyrene having the following elemental analysis.
References
Living cationic sequential block copolymerization of isobutylene with 4-tert-butoxystyrene: synthesis and characterization of poly(p-hydroxystyrene-b-isobutylene-b-p-hydroxystyrene) triblock copolymers.
Bouchékif, H.; Som, A.; Sipos, L.; Faust, R.; Journal of Macromolecular Science, Part A: Pure and Applied Chemistry (2007), 44(4), 359-366.
4-tert-Butoxystyrene Preparation Products And Raw materials
Raw materials
Preparation Products
| Supplier | Tel | Country | ProdList | Advantage | Inquiry |
|---|---|---|---|---|---|
| Aether Industries Limited | +91-261-6603130 +91-2616603130 | Gujarat, India | 145 | 58 | Inquiry |
| TCI Chemicals (India) Pvt. Ltd. | 1800 425 7889 | New Delhi, India | 6778 | 58 | Inquiry |
| Sholon Chem-Tech Co.,Ltd | +8619901505185 | China | 477 | 58 | Inquiry |
| Heynova (Shanghai) New Material Technology CO., Ltd | +86-17821320848; +8617821320848 | China | 431 | 58 | Inquiry |
| CHINATECH(TIANJIN) CHEMICAL CO.,LTD. | +86-18222270857 +86-18222265956 | China | 64 | 58 | Inquiry |
| Shanghai Joiny Pharmaceutical Co.,LTD | +8613681955282 | China | 249 | 58 | Inquiry |
| Shanghai Daken Advanced Materials Co.,Ltd | +86-371-66670886 | China | 16209 | 58 | Inquiry |
| career henan chemical co | +86-0371-86658258 +8613203830695 | China | 29900 | 58 | Inquiry |
| Shenzhen Nexconn Pharmatechs Ltd | +86-755-89396905 +86-15013857715 | China | 10260 | 58 | Inquiry |
| Chongqing Chemdad Co., Ltd | +86-023-6139-8061 +86-86-13650506873 | China | 39916 | 58 | Inquiry |





