Sodium sulfate
- CAS No.
- 7757-82-6
- Chemical Name:
- Sodium sulfate
- Synonyms
- Na2SO4;SODIUM SULPHATE;Sodium Sulphate Anhydrous;Anhydrous sodium sulfate;Sodium sulfate, anhydrous;anhydrous sodium sulphate;NaSO;Disodium sulphate;Mirabilite;Natriumsulfat
- CBNumber:
- CB4100946
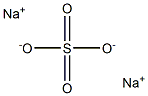
- Molecular Formula:
- Na2SO4
- Molecular Weight:
- 142.04214
- MOL File:
- 7757-82-6.mol
- MSDS File:
- SDS
- Modify Date:
- 2024/6/11 18:14:38
| Melting point | 884 °C (lit.) |
|---|---|
| Boiling point | 1700°C |
| Density | 2.68 g/mL at 25 °C (lit.) |
| refractive index | 1.484 |
| storage temp. | Store at +5°C to +30°C. |
| solubility | H2O: 1 M at 20 °C, clear, colorless |
| form | powder (fine) |
| Specific Gravity | 2.68 |
| color | White |
| PH | 5.2-8.0 (50g/l, H2O, 20℃) |
| PH Range | 5.2 - 9.2 |
| Odor | wh. cryst. or powd., odorless, bitter saline taste |
| Water Solubility | 18.5 mg/L |
| λmax |
λ: 260 nm Amax: 0.015 λ: 280 nm Amax: 0.015 |
| Sensitive | Hygroscopic |
| Merck | 14,8680 |
| Dielectric constant | 2.7(Ambient) |
| Stability | Stable. Incompatible with strong acids, aluminium, magnesium, strong bases. Hygroscopic. |
| LogP | -1.031 (est) |
| CAS DataBase Reference | 7757-82-6(CAS DataBase Reference) |
| NIST Chemistry Reference | Sodium sulfate(7757-82-6) |
| EPA Substance Registry System | Sodium sulfate (7757-82-6) |
SAFETY
Risk and Safety Statements
| Symbol(GHS) |  GHS07 |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Signal word | Warning | |||||||||
| Hazard statements | H317 | |||||||||
| Precautionary statements | P261-P272-P280-P302+P352-P333+P313-P321-P363-P501 | |||||||||
| Hazard Codes | Xi | |||||||||
| Risk Statements | 36/37/38 | |||||||||
| Safety Statements | 24/25 | |||||||||
| WGK Germany | 1 | |||||||||
| RTECS | WE1650000 | |||||||||
| F | 3 | |||||||||
| TSCA | Yes | |||||||||
| HS Code | 28331100 | |||||||||
| Toxicity | LD50 orally in Rabbit: > 2000 mg/kg | |||||||||
| NFPA 704 |
|
Sodium sulfate price More Price(95)
| Manufacturer | Product number | Product description | CAS number | Packaging | Price | Updated | Buy |
|---|---|---|---|---|---|---|---|
| Sigma-Aldrich(India) | S9627 | Sodium sulfate ReagentPlus?, ≥99.0% | 7757-82-6 | 500G | ₹6819.75 | 2022-06-14 | Buy |
| Sigma-Aldrich(India) | S9627 | Sodium sulfate ReagentPlus?, ≥99.0% | 7757-82-6 | 2.5KG | ₹9883.23 | 2022-06-14 | Buy |
| Sigma-Aldrich(India) | S9627 | Sodium sulfate ReagentPlus?, ≥99.0% | 7757-82-6 | 10KG | ₹21379.38 | 2022-06-14 | Buy |
| Sigma-Aldrich(India) | S6547 | Sodium sulfate BioXtra, ≥99.0% | 7757-82-6 | 500G | ₹5726.43 | 2022-06-14 | Buy |
| Sigma-Aldrich(India) | S6547 | Sodium sulfate BioXtra, ≥99.0% | 7757-82-6 | 2.5KG | ₹20751.53 | 2022-06-14 | Buy |
Sodium sulfate Chemical Properties,Uses,Production
Chemical Properties
Sodium sulfate, NaS04, also known as thenardite and salt cake, is a crystalline compound that melts at 888°C (1632°C). Sodium sulfate is found in natural form(thenardite) in Chile and Spain. It is used in the manufacture of paperboard, glass,and freezing mixtures. The hydrate, Na2S04·10H20, also known as "Glauber's salt," is a white water-soluble solid formed by heating sodium chloride and sulfuric acid. It is used in dyeing,manufacturing glass,and in the preparation of sodium bisulfate.
Physical properties
Anhydrous sodium sulfate is a white crystalline powder; orthorhombic or hexagonal structure; hygroscopic; refractive index 1.468; hardness 2.8 Mohs; density 2.664 g/cm3; melts at 884°C; soluble in water, insoluble in ethanol.
The decahydrate consists of colorless monoclinic crystals; refractive index 1.394; hardness 1.8 Mohs; density 1.4 6g/cm3; decomposes at 32°C; soluble in water; insoluble in ethanol.
Occurrence
Sodium sulfate occurs in nature as the minerals mirabilite and thenardite. While thenardite is the anhydrous form of Na2SO4, mirabilite is a naturallyoccurring decahydrate, Na2SO4?10H2O.
Sodium sulfate is one of the most important sodium salts. The decahydrate,commonly known as the Glauber’s salt, was first prepared by Johann Glauber in the seventeenth century as a by-product in making hydrochloric acid from sulfuric acid and sodium chloride.
Sodium sulfate is used in manufacturing paper pulp by the Kraft Process. Other uses are in manufacturing glass and ultramarine; in dyeing and printing textiles; as a filler in synthetic detergents; and for standardizing dyes. A major use of anhydrous sodium sulfate is as an agent to remove water from organic solvents and their extracts for organic synthesis and instrumental analysis. Sodium sulfate is a common laboratory reagent. Also, it is used to prepare other sodium salts.
Uses
Sodium sulfate is a filler in the manufacturing of synthetic detergents and soaps and a laboratory reagent. It may enhance the irritant action of certain detergents.
Production Methods
Sodium sulfate is mined from its natural mineral deposits and subjected to purification.
Sodium sulfate is synthesized by the Mannheim process or Hargreaves process. Manheim’s process is based on Glauber’s reaction between sulfuric acid and sodium chloride: 2NaCl + H2SO4 → Na2SO4 + 2HCl↑
The process was devised by Johann Glauber to produce hydrochloric acid. Sodium sulfate is isolated from the solution by fractional crystallization.
Hargreaves’ process also was developed to produce hydrochloric acid. It is a variation of Mannheim’s method. In this method, sulfur dioxide is used instead of sulfuric acid. The reaction is as follows: 4NaCl + 2SO2 + O2 + 2H2O → 2Na2SO4 + 4HCl↑
Sodium sulfate also is obtained as a byproduct of manufacturing phenol by caustic fusion.
Definition
A white crystalline compound, Na2SO4, usually known as the anhydrous compound (orthorhombic; r.d. 2.67; m.p. 888°C) or the decahydrate (monoclinic; r.d. 1.46; which loses water at 100°C). The decahydrate is known as Glauber’s salt. A metastable heptahydrate (Na2SO4·7H2O) also exists. All forms are soluble in water, dissolving to give a neutral solution. The compound occurs naturally as
mirabilite (Na2SO4·10H2O),
threnardite (Na2SO4), and
glauberite (Na2SO4·CaSO4).
Sodium sulphate may be produced industrially by the reaction of magnesium sulphate with sodium chloride in solution followed by crystallization, or by the reaction of concentrated sulphuric acid with solid sodium chloride. The latter method was used in the Leblanc process for the production of alkali and has given the name salt cake to impure industrial sodium sulphate. Sodium sulphate is used in the manufacture of glass and soft glazes and in dyeing to promote an even finish. It also finds medicinal application as a purgative and in commercial aperient salts.
General Description
Sodium sulfate (Na2SO4) can be obtained directly via precipitation from a saturated solution of Na2SO4 at room temperature (20°C). Its crystallization and growth rates at room temperature (20°C) under various conditions have been investigated. Sodium sulfate can be produced during Leblanc process, from sodium chloride and sulfuric acid.
Safety Profile
Moderately toxic by intravenous route. Mildly toxic by ingestion. An experimental teratogen. Experimental reproductive effects. Questionable carcinogen with experimental tumorigenic effects. Violent reaction with Al. When heated to decomposition it emits toxic fumes of SOx and Na2O. See also SULFATES.
Potential Exposure
Sodium sulfate is used in the manufacture of glass; as a precipitating agent in the manufacture of silver emulsions; as an analytical reagent; in making ultramarine and paper pulp; in ceramic glazes and pharmaceuticals; as a food additive; and a filler in synthetic detergents.
Shipping
UN2630 Selenates or Selenites, Hazard Class: 6.1; Labels: 6.1-Poisonous materials.
Purification Methods
Crystallise sodium sulfate from water at 30o (1.1mL/g) by cooling to 0o. It becomes anhydrous at 32o.
Incompatibilities
Violent reaction with aluminum, magnesium. Attacks metals in the presence of moisture.
Waste Disposal
Do not discharge waste sodium sulfate directly into sewers or surface waters. Recovered sodium sulfate may be disposed of by burial in a landfill.
Sodium sulfate Preparation Products And Raw materials
Raw materials
1of2
chevron_rightPreparation Products
1of8
chevron_right| Supplier | Tel | Country | ProdList | Advantage | Inquiry |
|---|---|---|---|---|---|
| ANJI BIOSCIENCES | +91-9000100077 +91-9000100077 | Hyderabad, India | 430 | 58 | Inquiry |
| SUKHA CHEMICAL INDUSTRIES | +91-9638875444 +91-9638875444 | Gujarat, India | 26 | 58 | Inquiry |
| Nikava Pharmaceutical Industries | +91-9653317212 +91-9653317212 | Mumbai, India | 20 | 58 | Inquiry |
| Vardhaman P Golechha | 9704422000 | Telangana, India | 294 | 58 | Inquiry |
| SNECOFRi Pvt Ltd | +91-9032850129 +91-9032850129 | Telangana, India | 404 | 58 | Inquiry |
| JSK Chemicals | +919879767970 | Gujarat, India | 3756 | 58 | Inquiry |
| SOLFYN INTERNATIONAL LLP | +91-9321772608 +91-9321772608 | Mumbai, India | 105 | 58 | Inquiry |
| Kronox Lab Sciences Pvt Ltd | +91-9313231074 +91-9313231074 | Gujarat, India | 240 | 58 | Inquiry |
| Sree Rayalaseema HI Strength Hypo Ltd | +91-9848867692 +91-9848867692 | Andhra Pradesh, India | 19 | 58 | Inquiry |
| Atul Ltd | +91-2362230000 +91-2362230000 | Gujarat, India | 26 | 58 | Inquiry |
| Supplier | Advantage |
|---|---|
| ANJI BIOSCIENCES | 58 |
| SUKHA CHEMICAL INDUSTRIES | 58 |
| Nikava Pharmaceutical Industries | 58 |
| Vardhaman P Golechha | 58 |
| SNECOFRi Pvt Ltd | 58 |
| JSK Chemicals | 58 |
| SOLFYN INTERNATIONAL LLP | 58 |
| Kronox Lab Sciences Pvt Ltd | 58 |
| Sree Rayalaseema HI Strength Hypo Ltd | 58 |
| Atul Ltd | 58 |
Related articles
- Sodium sulfate: Physical Properties and Uses
- Sodium sulfate has unusual solubility characteristics in water and has different uses.
- Nov 16,2022
Related Qustion
- Q:What is the mechanism of Sodium sulfate as a laxative?
- A:An oral formulation containing Sodium sulfate having osmotic laxative activity.
- Apr 23,2024
- Q:What is the toxicity of Sodium sulfate
- A:Sodium sulfate may result in gastrointestinal irritation and diarrhea.
- Dec 25,2023





