Ammonium sulfate
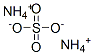
- CAS No.
- 7783-20-2
- Chemical Name:
- Ammonium sulfate
- Synonyms
- AMMONIUM SULPHATE;(NH4)2 SO4;AMMNIUM SULPHATE;AMMONIUM SULPHAT;Liase;dolamin;nsc77671;actamaster;mascagnite;Ammonium suL
- CBNumber:
- CB9466357
- Molecular Formula:
- H8N2O4S
- Molecular Weight:
- 132.14
- MOL File:
- 7783-20-2.mol
- MSDS File:
- SDS
- Modify Date:
- 2024/6/14 8:38:41
| Melting point | >280 °C (dec.) (lit.) |
|---|---|
| Density | 1.77 g/mL at 25 °C (lit.) |
| vapor pressure | <1 Pa (25 °C) |
| refractive index |
n |
| Flash point | 26 °C |
| storage temp. | room temp |
| solubility | H2O: 1 M at 20 °C, clear, colorless |
| form | Solid |
| Specific Gravity | 1.769 |
| color | Yellow to orange |
| PH | 5.0-6.0 (25℃, 1M in H2O) |
| Odor | Slight odor of ammonia |
| PH Range | 5 - 6 |
| Water Solubility | 77 g/100 mL (25 ºC) |
| λmax |
λ: 260 nm Amax: ≤0.037 λ: 280 nm Amax: ≤0.030 |
| Merck | 14,555 |
| Stability | Stable. Contact with strong oxidizers may cause fire or explosion. Incompatible with strong bases. |
| InChIKey | BFNBIHQBYMNNAN-UHFFFAOYSA-N |
| LogP | -5.1 at 25℃ |
| CAS DataBase Reference | 7783-20-2(CAS DataBase Reference) |
| EPA Substance Registry System | Ammonium sulfate (7783-20-2) |
SAFETY
Risk and Safety Statements
| Symbol(GHS) |  GHS07 |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Signal word | Warning | |||||||||
| Hazard statements | H412-H303 | |||||||||
| Precautionary statements | P273 | |||||||||
| Hazard Codes | Xi,Xn | |||||||||
| Risk Statements | 10-36/37/38-22 | |||||||||
| Safety Statements | 37/39-26-36-24/25 | |||||||||
| WGK Germany | 1 | |||||||||
| RTECS | BS4500000 | |||||||||
| TSCA | Yes | |||||||||
| HS Code | 31022100 | |||||||||
| Toxicity | LD50 orally in Rabbit: 2840 mg/kg | |||||||||
| NFPA 704 |
|
Ammonium sulfate price More Price(89)
| Manufacturer | Product number | Product description | CAS number | Packaging | Price | Updated | Buy |
|---|---|---|---|---|---|---|---|
| Sigma-Aldrich(India) | A4915 | Ammonium sulfate ACS reagent, ≥99.0% | 7783-20-2 | 500G | ₹3366.58 | 2022-06-14 | Buy |
| Sigma-Aldrich(India) | A4915 | Ammonium sulfate ACS reagent, ≥99.0% | 7783-20-2 | 25G | ₹4265.05 | 2022-06-14 | Buy |
| Sigma-Aldrich(India) | A5132 | Ammonium sulfate ReagentPlus?, ≥99.0% | 7783-20-2 | 1KG | ₹3518.13 | 2022-06-14 | Buy |
| Sigma-Aldrich(India) | A4915 | Ammonium sulfate ACS reagent, ≥99.0% | 7783-20-2 | 1KG | ₹11258 | 2022-06-14 | Buy |
| Sigma-Aldrich(India) | A4915 | Ammonium sulfate ACS reagent, ≥99.0% | 7783-20-2 | 2.5KG | ₹21888.15 | 2022-06-14 | Buy |
Ammonium sulfate Chemical Properties,Uses,Production
Description
Ammonium sulfate was the first
nitrogenous fertilizer made by the Haber-Bosch process,
produced by the reaction of ammonia with sulfuric acid. In
contrast with the nitrate salt, it is chemically stable, not
highly hygroscopic. It also supplies supplemental sulfur to
soils that may be deficient in this element, but this is of
minor value when it is used on soils receiving applications
of ordinary superphosphate.
The disadvantages of the
material are its relatively low nitrogen content, which
increases storage and transportation costs, and its marked
tendency to cause soil acidification, which is greater than
that of any other nitrogen fertilizer material.
Chemical Properties
White crystalline powder
Physical properties
White crystalline solid; orthorhombic crystal; density 1.769 g/cm3 at 20°C; melts between 511 to 515°C (in a closed system): however, in an open system, it melts with decomposition at 280°C; readily dissolves in water (solubility, 70.6 g and 104 g per 100 g water at 0°C and 100°C, respectively); insoluble in acetone, alcohol and ether.
Occurrence
Ammonium sulfate occurs in trace concentrations in the upper atmosphere. It is widely used as a fertilizer for rice and other crops. It is a source of sulfur for the soil. It is also used as an additive to supply nutrient nitrogen in fermentation processes (e.g., yeast production from molasses). It also is used for fireproofing timber and plastics, and in treatment of hides, and leather production.
Uses
Ammonium Sulfate is a dough conditioner, firming agent, and pro- cessing aid which is readily soluble in water with a solubility of approximately 70 g in 100 g of water at 0°c. the ph of a 0.1 molar solution in water is approximately 5.5. it is used in caramel produc- tion and as a source of nitrogen for yeast fermentation. in bakery products, up to 0.25 part per 100 parts by weight of flour is used.
Definition
ammonium sulphate: A whiterhombic solid, (NH4)2SO4; r.d. 1.77;decomposes at 235°C. It is very solublein water and insoluble in ethanol.It occurs naturally as the mineralmascagnite. Ammonium sulphatewas formerly manufactured from the‘ammoniacal liquors’ produced duringcoal-gas manufacture but is nowproduced by the direct reaction betweenammonia gas and sulphuricacid. It is decomposed by heating torelease ammonia (and ammoniumhydrogensulphate) and eventuallywater, sulphur dioxide, and ammonia.Vast quantities of ammoniumsulphate are used as fertilizers.
Production Methods
Ammonium sulfate is a high-tonnage industrial chemical, but frequently may be considered a byproduct as well as intended end-product of manufacture. A significant commercial source of (NH4)2SO4 is its creation as a byproduct in the manufacture of caprolactam, which yields several tons of the compound per ton of caprolactam made. Ammonium sulfate also is a byproduct of coke oven operations where the excess NH3 formed is neutralized with H2SO4 to form (NH4)2SO4. In the Meresburg reaction, natural or byproduct gypsum is reacted with ammonium carbonate: CaSO4·2H2O + (NH4)2CO3 CaCO3 + (NH4)2SO4 +2 H2O The product is stable, free-flowing crystals. As a fertilizer, (NH4)2SO4 has the advantage of adding sulfur to the soil as well as nitrogen. By weight, the compound contains 21% N and 24% S. Ammonium sulfate also is used in electric dry cell batteries, as a soldering liquid, as a fire retardant for fabrics and other products, and as a source of certain ammonium chemicals.
General Description
White odorless solid. Sinks and dissolves in water.
Air & Water Reactions
Dissolves in water with evolution of some heat.
Reactivity Profile
Ammonium sulfate is acidic in aqueous solution. When a little Ammonium sulfate is added to fused potassium nitrite, a vigorous reaction occurs attended by flame [Mellor 2:702 1946-47].
Safety Profile
Moderately toxic by several routes. Human systemic effects by ingestion: hypermotility, diarrhea, nausea or vomiting. See also SULFATES. Incandescent reaction on heating with potassium chlorate. Reaction with sodmm hypochlorite gves the unstable explosive nitrogen trichloride. Incompatible with (K + NH4NO3), KNO2, (NaK + NH4NO3). When heated to decomposition it emits very toxic fumes of NOx, NH3, and SOx.
Purification Methods
Crystallise it twice from hot water containing 0.2% EDTA to remove metal ions, then finally from distilled water. Dry it in a desiccator for 2 weeks over Mg(ClO4)2. After 3 recrystallisations, ACS grade had Ti, K, Fe, Na at 11, 4.4, 4.4, 3.2 ppm respectively.
Ammonium sulfate Preparation Products And Raw materials
Raw materials
1of2
chevron_rightPreparation Products
1of8
chevron_right| Supplier | Tel | Country | ProdList | Advantage | Inquiry |
|---|---|---|---|---|---|
| GLR Innovations | +91 9891111994 | New Delhi, India | 4542 | 58 | Inquiry |
| Rishi Chemical Works Pvt Ltd | +91-3322290068 +91-3322290071 | Kolkata, India | 43 | 58 | Inquiry |
| Yogi Dye Chem Industries | +91-9821098577 +91-9821098577 | Mumbai, India | 124 | 58 | Inquiry |
| Indiana ChemPort | +91-91-2652638667 +91-7574886711 | Gujarat, India | 85 | 58 | Inquiry |
| QUALIKEMS FINE CHEM PVT LTD | +911123618475 | New Delhi, India | 51 | 58 | Inquiry |
| ADVENT CHEMBIO PRIVATE LIMITED | +91-9821524116 +91-9821524116 | Mumbai, India | 353 | 58 | Inquiry |
| Choice Chemicals | +91-9895031268 +91-9895031268 | Kerela, India | 118 | 58 | Inquiry |
| JSK Chemicals | +919879767970 | Gujarat, India | 3756 | 58 | Inquiry |
| UNILOSA INTERNATINAL PRIVATE LIMITED | +91-9999069917 +91-9999069917 | New Delhi, India | 152 | 58 | Inquiry |
| AVA CHEMICALS PVT LTD | +91-8879390046 +91-8879390046 | Maharashtra, India | 74 | 58 | Inquiry |
| Supplier | Advantage |
|---|---|
| GLR Innovations | 58 |
| Rishi Chemical Works Pvt Ltd | 58 |
| Yogi Dye Chem Industries | 58 |
| Indiana ChemPort | 58 |
| QUALIKEMS FINE CHEM PVT LTD | 58 |
| ADVENT CHEMBIO PRIVATE LIMITED | 58 |
| Choice Chemicals | 58 |
| JSK Chemicals | 58 |
| UNILOSA INTERNATINAL PRIVATE LIMITED | 58 |
| AVA CHEMICALS PVT LTD | 58 |
Related articles
- Food Grade, Electronic Grade, and Reagent Grade A Detailed Introduction of Ammonium sulfate
- Ammonium sulfate is often subdivided into food grade, electronic grade, and reagent grade based on its purity.
- Jun 14,2024
- Synthesis of Ammonium sulfate
- Ammonium sulfate is an inorganic sulfate salt obtained by reaction of sulfuric acid with two equivalents of ammonia. A high-me....
- Mar 31,2022
- Uses of Ammonium sulfate
- Ammonium sulfate is an inorganic sulfate salt obtained by reaction of sulfuric acid with two equivalents of ammonia,it is wide....
- Feb 21,2022
7783-20-2(Ammonium sulfate)Related Search:
1of4
chevron_right




