Ammonium nitrate
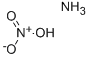
- CAS No.
- 6484-52-2
- Chemical Name:
- Ammonium nitrate
- Synonyms
- Ammoniumnitrat;AMMONIA NITRATE;nitram;varioformi;hercoprills;mercoprills;caswellno.045;nitratoamonico;germansaltpeter;Azanium nitrate
- CBNumber:
- CB4167347
- Molecular Formula:
- H4N2O3
- Molecular Weight:
- 80.04
- MOL File:
- 6484-52-2.mol
- Modify Date:
- 2023/4/23 13:52:06
| Melting point | 169 °C(lit.) |
|---|---|
| Boiling point | 210 °C(lit.) |
| Density | 1.72 |
| Flash point | 210°C |
| storage temp. | Store at RT. |
| solubility | H2O: 1 M at 20 °C, clear, colorless |
| form | Solid |
| Specific Gravity | 1.725 |
| color | White |
| PH | 4.5-6.0 (25℃, 50mg/mL in H2O) |
| Water Solubility | 190 g/100 mL (20 ºC) |
| Merck | 14,534 |
| CAS DataBase Reference | 6484-52-2(CAS DataBase Reference) |
| EPA Substance Registry System | Ammonium nitrate (6484-52-2) |
SAFETY
Risk and Safety Statements
| Symbol(GHS) |   GHS03,GHS07 |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Signal word | Warning | |||||||||
| Hazard statements | H272-H319 | |||||||||
| Precautionary statements | P210-P220-P264-P280-P305+P351+P338-P337+P313 | |||||||||
| Hazard Codes | O,Xi | |||||||||
| Risk Statements | 8-36/37/38-9 | |||||||||
| Safety Statements | 17-26-36-37/39-41-16-15 | |||||||||
| RIDADR | UN 1942 5.1/PG 3 | |||||||||
| WGK Germany | 1 | |||||||||
| RTECS | BR9050000 | |||||||||
| F | 3-10 | |||||||||
| TSCA | Yes | |||||||||
| HazardClass | 5.1 | |||||||||
| PackingGroup | III | |||||||||
| HS Code | 31023090 | |||||||||
| Toxicity | LD50 orally in Rabbit: 2462 mg/kg | |||||||||
| NFPA 704 |
|
Ammonium nitrate Chemical Properties,Uses,Production
Description
Ammonium nitrate is an oxidizer, which may explode under confinement and high temperatures. When mixed with fuel oil, a deflagrating explosive material is created. Ammonium nitrate and fuel oil were used as the explosive in the Alfred P. Murrah Federal Building terrorist attack in Oklahoma City and the first terror attack on the World Trade Center in New York City in the mid-1990s.
Chemical Properties
Ammonium nitrate,NH4N03, is a colorless crystalline solid existing in two forms, Between 16 and 32°C, the crystals are tetragonal; between 32 and 84 DC, the crystals are rhombic. The melting point of NH4N03 is 169.6 DC, and it decomposes above 210°C. When heated, ammonium nitrate yields nitrous oxide gas. Ammonium nitrate is soluble in water, in acetic acid solutions containing ammonia, is slightly soluble in ethanol, and is moderately soluble in methanol.

Ammonium nitrate is a very insensitive and stable high explosive used as a slow-burning propellant for rockets when compounded with burning rate catalysts. Although the major applications of Ammonium nitrate are explosives and fertilizers, it is also used in insecticides, rust inhibitors, and pyrotechnics.
Uses
Ammonium nitrate (NH4NO3, also known as “Norway saltpeter”) is mainly used as a fertilizer. It is also known as the chemical that was mixed with diesel fuel to create the explosion that demolished the Murrah Federal Building in Oklahoma City in 1995.
Definition
ammonium nitrate: A colourlesscrystalline solid, NH4NO3; r.d. 1.72;m.p. 169.6°C; b.p. 210°C. It is verysoluble in water and soluble inethanol. The crystals are rhombicwhen obtained below 32°C andmonoclinic above 32°C. It may bereadily prepared in the laboratory bythe reaction of nitric acid with aqueousammonia. Industrially, it is manufacturedby the same reaction usingammonia gas. Vast quantities of ammoniumnitrate are used as fertilizers(over 20 million tonnes per year)and it is also a component of someexplosives.
General Description
A colorless crystalline solid. Soluble in water. Does not readily burn but will do so if contaminated with combustible material. Accelerates the burning of combustible material. Produces toxic oxides of nitrogen during combustion. Used to make fertilizers and explosives, and as a nutrient in producing antibiotics and yeast.
Air & Water Reactions
Water soluble. Hot aqueous solutions of the nitrate above 50% conc., under confinement may decompose explosively. This process is aided catalytically with such substances as nitric acid and chloride ion, [Chem. Abs., 1982, 97, 78074].
Reactivity Profile
The hazards of AMMONIUM NITRATE have been well studied because of several extremely severe explosions [Chem. Eng., 1962, 70, 91; Bretherick, 5th Ed., 1995]. Mixtures with alkyl esters may explode, owing to the formation of alkyl nitrates. Mixtures with phosphorus, tin(II) chloride or other reducing agents may react explosively [Bretherick 1979 p. 108-109]. A mixture with aluminum powder (also zinc, cadmium, copper, magnesium, lead, cobalt, nickel, bismuth, chromium, and antimony) can be used as an explosive. A number of explosions in which ammonium nitrate and aluminum were mixed with carbon or hydrocarbons, with or without oxidizing agents have occurred [Mellor 5:219 1946-47]. A mixture with acetic acid ignites when warmed, especially if concentrated [Von Schwartz p. 322 1918]. Causes the decomposition of sodium hypochlorite within a few seconds [Mellor 2 Supp. 1:550 1956].
Hazard
May explode under confinement and high temperatures, but not readily detonated. Ventilate well. To fight fire, use large amounts of water. The material must be kept as cool as possible and removed from confinement and flooded with water in event of fire.
Health Hazard
Inhalation, ingestion or contact (skin, eyes) with vapors or substance may cause severe injury, burns or death. Fire may produce irritating, corrosive and/or toxic gases. Runoff from fire control or dilution water may cause pollution.
Fire Hazard
These substances will accelerate burning when involved in a fire. Some may decompose explosively when heated or involved in a fire. May explode from heat or contamination. Some will react explosively with hydrocarbons (fuels). May ignite combustibles (wood, paper, oil, clothing, etc.). Containers may explode when heated. Runoff may create fire or explosion hazard.
Potential Exposure
Used in the manufacture of liquid and solid fertilizer compositions, industrial explosives and blasting agents from ammonium nitrate, matches; antibiotics; in the production of nitrous oxide.
Metabolism
Ammonium Nitrate. Ammonium nitrate is one of the two leading nitrogen fertilizer materials on a world basis: 10% in 1997. The high N content is advantageous for the reduction of freight and application costs per unit weight of nitrogen. The presence of 50% of the nitrogen in the highly available nitrate form makes it suitable for use in regions growing crops with a short vegetation period but has the disadvantage that, because the NO3 ? ion is not adsorbed by soil, it may contribute to relatively large nitrogen losses by the leaching of increased soil nitrate into streams and groundwater. Although the application of any nitrogenous fertilizer results in some degree of soil acidification, the nitrate form is notably less acidifying than ammonium sulfate and has a lower tendency for the loss of nitrogen to the atmosphere as gaseous ammonia. The hygroscopic character of the crystalline material, coupled with its explosive nature, contributes to difficult storage and handling properties and the need for the production of purified and stabilized forms.
Shipping
Ammonium nitrate with organic coating: UN0222 Ammonium nitrate, with . 0.2% combustible substances, including any organic substance calculated as carbon, to the exclusion of any other added substance, Hazard Class: 1D; Labels:1D-Explosive (with a mass explosion hazard); D-Substances or articles which may mass detonate (with blast and/or fragment hazard) when exposed to fire. Ammonium nitrate with NO organic coating: UN1942 Ammonium nitrate, with NOT . 0.2% of combustible substances, including any organic substance calculated as carbon, to the exclusion of any other added substance (also used for fertilizer), Hazard Class: 5.1; Labels: 5.1-Oxidizer. UN3375 Ammonium nitrate emulsion or Ammonium nitrate suspension or Ammonium nitrate gel, intermediate for blasting explosives, Hazard Class: 5.1; Labels: 5.1-Oxidizer. UN2072 Ammonium nitrate fertilizer, n.o.s., doesn’t appear in the 49 CFR Hazmat Table, refer to UN1942, above). UN2071 Ammonium nitrate based fertilizer, Hazard class: 9; Labels: 9-Miscellaneous hazardous material. UN2426/140 Ammonium nitrate, liquid (hot concentrated solution), Hazard Class: 5.1; Labels: 5.1-Oxidizer.
Purification Methods
It is crystallised twice from distilled water (1mL/g) by adding EtOH, or from warm water (0.5mL/g) by cooling in an ice-salt bath. Dry it in air, then under vacuum. After 3 recrystallisations of ACS grade, it contained Li and B at 0.03 and 0.74 ppm, respectively. It is deliquescent. [Early & Lowry J Chem Soc 115 1387 1919, 121 963 1922, Hendricks et al. J Am Chem Soc 54 2766 1932.]
Incompatibilities
A strong oxidizer. Reducing agents; combustible materials; organic materials; finely divided (powdered) metals may form explosive mixtures or cause fire and explosions. When contaminated with oil, charcoal or flammable liquids, can be considered an explosive which can be detonated by combustion or shock.
Waste Disposal
Pretreatment involves addition of sodium hydroxide to liberate ammonia and form the soluble sodium salt. The liberated ammonia can be recovered and sold. After dilution to the permitted provisional limit, the sodium salt can be discharged into a stream or sewer.
Ammonium nitrate Preparation Products And Raw materials
Raw materials
1of2
chevron_rightPreparation Products
1of7
chevron_right6484-52-2(Ammonium nitrate)Related Search:
1of4
chevron_right




