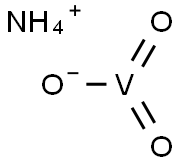
Ammonium metavanadate
- CAS No.
- 7803-55-6
- Chemical Name:
- Ammonium metavanadate
- Synonyms
- NH4VO3;VANADIUM OXIDE;AMMONIUM VANADATE;Ammoniun metavanabate;ammonium trioxovanadate;AMMONIUM VANADIUM OXIDE;Ammonium vanadium oxide, ACS, 99.0% min;Ammoniumv;HSDB 6310;ACS, (RT)
- CBNumber:
- CB8854364
- Molecular Formula:
- H4NO3V
- Molecular Weight:
- 116.98
- MOL File:
- 7803-55-6.mol
- MSDS File:
- SDS
- Modify Date:
- 2024/7/4 14:06:19
| Melting point | 200 °C |
|---|---|
| Density | 2.32 g/cm3 at 25 °C (lit.) |
| storage temp. | Store at RT. |
| solubility | H2O: soluble |
| form | Solid |
| Specific Gravity | 2.33 |
| color | White to yellow |
| Odor | Odorless |
| PH | 7 (5.1g/l, H2O, 20℃) |
| Water Solubility | 5.1 g/L (20 ºC) |
| Merck | 14,568 |
| Exposure limits | NIOSH: Ceiling 0.05 mg/m3 |
| Stability | Stable. Incompatible with strong acids, strong oxidizing agents. Protect from moisture - hygroscopic. |
| CAS DataBase Reference | 7803-55-6(CAS DataBase Reference) |
| EPA Substance Registry System | Ammonium vanadate (7803-55-6) |
SAFETY
Risk and Safety Statements
| Symbol(GHS) |    GHS06,GHS08,GHS09 |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Signal word | Danger | |||||||||
| Hazard statements | H301-H319-H332-H372-H411 | |||||||||
| Precautionary statements | P273-P301+P310+P330-P304+P340+P312-P305+P351+P338-P314 | |||||||||
| Hazard Codes | T+,T | |||||||||
| Risk Statements | 25-26-36/37/38-20-34-22-36/37-23/24/25 | |||||||||
| Safety Statements | 26-36/37/39-45-37/39-22-28 | |||||||||
| RIDADR | UN 2859 6.1/PG 2 | |||||||||
| WGK Germany | 3 | |||||||||
| RTECS | YW0875000 | |||||||||
| F | 3 | |||||||||
| TSCA | Yes | |||||||||
| HazardClass | 6.1 | |||||||||
| PackingGroup | II | |||||||||
| HS Code | 28419030 | |||||||||
| Toxicity | LD50 orally in rats: 0.16 g/kg (Smyth) | |||||||||
| NFPA 704 |
|
Ammonium metavanadate price More Price(30)
| Manufacturer | Product number | Product description | CAS number | Packaging | Price | Updated | Buy |
|---|---|---|---|---|---|---|---|
| Sigma-Aldrich(India) | 398128 | Ammonium metavanadate ACS reagent, ≥99.0% | 7803-55-6 | 50G | ₹3972.78 | 2022-06-14 | Buy |
| Sigma-Aldrich(India) | 398128 | Ammonium metavanadate ACS reagent, ≥99.0% | 7803-55-6 | 250G | ₹11712.65 | 2022-06-14 | Buy |
| Sigma-Aldrich | 205559 | Ammonium metavanadate 99% | 7803-55-6 | 250G | ₹11073.98 | 2022-06-14 | Buy |
| Sigma-Aldrich(India) | 205559 | Ammonium metavanadate 99% | 7803-55-6 | 250G | ₹11073.98 | 2022-06-14 | Buy |
| Sigma-Aldrich | 205559 | Ammonium metavanadate 99% | 7803-55-6 | 1KG | ₹14862.73 | 2022-06-14 | Buy |
Ammonium metavanadate Chemical Properties,Uses,Production
Chemical Properties
Ammonium metavanadate is an inorganic acidic salt with the formula NH4VO3. It is a white or slightly yellow crystalline powder. Slightly soluble in cold water, hot ethanol and ether, soluble in hot water and dilute ammonium hydroxide. It is an important intermediate in the purification of vanadium.
Uses
Ammonium vanadium oxide is used as a catalyst in organic synthesis, and as an analytical reagent in laboratories. It is employed in the preparation of vanadate. It acts as a sensor for the determination of N-acetyltransferase activity in humans. Further it finds use in dyes, varnishes, indelible inks, drier for paints, and in photography. With copper carbonate, it has been shown to enhance the catalytic activities. Ammonium metavanadate is an environmental-friendly catalyst to prepare alpha-hydroxyphosphonate derivatives in high yield through the reaction of aryl/heteroaryl aldehydes with triethylphosphonate derivatives.
General Description
Ammonium metavanadate appears as a white crystalline powder. Slightly soluble in water and denser than water. Decomposes at 410°F. May release toxic fumes. Moderately toxic. An irritant. Used as a dryer for paints and inks, and for dyes. Loses ammonia upon heating.
Air & Water Reactions
Slightly soluble in water.
Reactivity Profile
Acidic inorganic salts, such as Ammonium metavanadate, are generally soluble in water. The resulting solutions contain moderate concentrations of hydrogen ions and have pH's of less than 7.0. They react as acids to neutralize bases. These neutralizations generate heat, but less or far less than is generated by neutralization of inorganic acids, inorganic oxoacids, and carboxylic acid. They usually do not react as either oxidizing agents or reducing agents but such behavior is not impossible. Many of these compounds catalyze organic reactions.
Health Hazard
TOXIC; inhalation, ingestion or skin contact with material may cause severe injury or death. Contact with molten substance may cause severe burns to skin and eyes. Avoid any skin contact. Effects of contact or inhalation may be delayed. Fire may produce irritating, corrosive and/or toxic gases. Runoff from fire control or dilution water may be corrosive and/or toxic and cause pollution.
Fire Hazard
Non-combustible, substance itself does not burn but may decompose upon heating to produce corrosive and/or toxic fumes. Some are oxidizers and may ignite combustibles (wood, paper, oil, clothing, etc.). Contact with metals may evolve flammable hydrogen gas. Containers may explode when heated.
Safety Profile
Poison by ingestion, subcutaneous, intravenous, intratracheal, and intraperitoneal routes. Moderately toxic by skin contact. An experimental teratogen. Other experimental reproductive effects. Mutation data reported. See also VANADIUM COMPOUNDS. When heated to decomposition it emits toxic fumes of NH3, VOx, and NOx.
Potential Exposure
It is used in the metals industry to make alloys, chemical reactions, dyes, inks, varnishes, printing, medicines, and photography.
Shipping
UN2859 Ammonium metavanadate, Hazard Class: 6.1; Labels: 6.1-Poisonous materials.
Purification Methods
Wash the salt with H2O until free from Cl-ions and dry it in air. It is soluble in H2O (5.18g/100mL at 15o, 10.4g/100mL at 32o) but is more soluble in dilute NH3. It crystallises from conductivity water (20mL/g). When heated at relatively low temperature, it loses H2O and NH3 to give vanadium oxide (V2O5), and at 210o it forms lower oxides. [Baker et al. Inorg Synth III 117 1950.] Its solubility in H2O is 0.52% (15o), 1% (32o) and 1.6% (50o). After washing the technical grade salt with H2O, it had Na, Mn and U at 0.06, 0.2 and 0.1 ppm, respectively. [Brauer in Handbook of Preparative Inorganic Chem (Ed. Brauer) Academic Press Vol II p 1272-1273 1965.]
Incompatibilities
Moisture forms an acidic solution. React with bases and alkaline material: may generate heat and release flammable hydrogen gas.
Ammonium metavanadate Preparation Products And Raw materials
Raw materials
1of2
chevron_rightPreparation Products
| Supplier | Tel | Country | ProdList | Advantage | Inquiry |
|---|---|---|---|---|---|
| GLR Innovations | +91 9891111994 | New Delhi, India | 4542 | 58 | Inquiry |
| JSK Chemicals | +919879767970 | Gujarat, India | 3756 | 58 | Inquiry |
| Triveni chemicals | 08048762458 | New Delhi, India | 6093 | 58 | Inquiry |
| Cynor Laboratories | 08046057119 | Gujarat, India | 64 | 58 | Inquiry |
| SANA CHEM AND PHARMA | 08048361299Ext 617 | Mumbai, India | 11 | 58 | Inquiry |
| Zama Chemical | 08048983954 | Mumbai, India | 217 | 58 | Inquiry |
| A. B. Enterprises | +91-8048077585 | Mumbai, India | 1396 | 58 | Inquiry |
| Innovative | 07942565925 | Mumbai, India | 617 | 58 | Inquiry |
| Yogi Dye Chem Pvt Ltd. (Unit Of Forbes... | 08047637783 | Mumbai, India | 43 | 58 | Inquiry |
| Alliance Global | 08048965751 | Delhi, India | 211 | 58 | Inquiry |
| Supplier | Advantage |
|---|---|
| GLR Innovations | 58 |
| JSK Chemicals | 58 |
| Triveni chemicals | 58 |
| Cynor Laboratories | 58 |
| SANA CHEM AND PHARMA | 58 |
| Zama Chemical | 58 |
| A. B. Enterprises | 58 |
| Innovative | 58 |
| Yogi Dye Chem Pvt Ltd. (Unit Of Forbes... | 58 |
| Alliance Global | 58 |
Related articles
- Synthesis of Ammonium metavanadate
- The NH4VO3 can be recrystallized under similar conditions.
- Jul 4,2024
7803-55-6(Ammonium metavanadate)Related Search:
1of4
chevron_right




