Aminoacylase
- CAS No.
- 9012-37-7
- Chemical Name:
- Aminoacylase
- Synonyms
- ACYLASE;ACYLASE I;L-AMINOACYLASE;ACYLASE 1;histozyme;hippurase;acyclase I;EC 3.5.1.14;hippuricase;benzamidase
- CBNumber:
- CB3458624
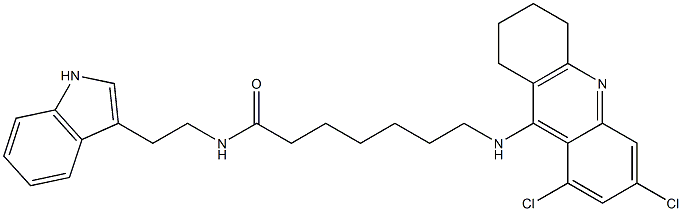
- Molecular Formula:
- C30H34Cl2N4O
- Molecular Weight:
- 537.52316
- MOL File:
- 9012-37-7.mol
- Modify Date:
- 2023/4/23 13:52:06
| storage temp. | 2-8°C |
|---|---|
| form | salt-free, lyophilized powder |
| color | yellow-brown |
| EPA Substance Registry System | Aminoacylase (9012-37-7) |
SAFETY
Risk and Safety Statements
| Symbol(GHS) |   GHS07,GHS08 |
|---|---|
| Signal word | Danger |
| Hazard statements | H315-H319-H334-H335 |
| Precautionary statements | P261-P264-P271-P302+P352-P304+P340+P312-P305+P351+P338 |
| Hazard Codes | Xn |
| Risk Statements | 36/37/38-42 |
| Safety Statements | 22-24/25-36/37-26-24 |
| WGK Germany | 3 |
| RTECS | BF4890000 |
| F | 3-10-21 |
| HS Code | 35079090 |
Aminoacylase price More Price(16)
| Manufacturer | Product number | Product description | CAS number | Packaging | Price | Updated | Buy |
|---|---|---|---|---|---|---|---|
| Sigma-Aldrich(India) | A3010 | Acylase I from porcine kidney Grade I, lyophilized powder, ≥1500?units/mg protein | 9012-37-7 | 100MG | ₹9557.1 | 2022-06-14 | Buy |
| Sigma-Aldrich(India) | A8376 | Acylase I from porcine kidney Grade II, salt-free, lyophilized powder, 300-1,500?units/mg protein | 9012-37-7 | 1G | ₹13819.5 | 2022-06-14 | Buy |
| Sigma-Aldrich(India) | A3010 | Acylase I from porcine kidney Grade I, lyophilized powder, ≥1500?units/mg protein | 9012-37-7 | 500MG | ₹31945.8 | 2022-06-14 | Buy |
| Sigma-Aldrich(India) | A8376 | Acylase I from porcine kidney Grade II, salt-free, lyophilized powder, 300-1,500?units/mg protein | 9012-37-7 | 10G | ₹112087.8 | 2022-06-14 | Buy |
| Sigma-Aldrich(India) | A3010 | Acylase I from porcine kidney Grade I, lyophilized powder, ≥1500?units/mg protein | 9012-37-7 | 1G | ₹49106.4 | 2022-06-14 | Buy |
Aminoacylase Chemical Properties,Uses,Production
Description
Aminoacylase-1 (EC 3.5.1.14) is a homodimeric zinc-binding metalloenzyme. A cytosolic enzyme with a wide range of tissue expression, it cleaves acylated L-amino acids (except L-aspartate) into L-amino acids and an acyl group. L-aspartate derivatives are cleaved by aminoacylase-2 (aspartoacylase). Aminoacylase-1 is the most abundant of the aminoacylases, a class of enzymes involved in hydrolysis of N-acetylated proteins.
Uses
Acylase I from porcine kidney has been used to study the acylase I-catalyzed deacetylation of various S-alkyl-N-acetyl-L-cysteines and their carbon and oxygen analogues . Acylase I may be useful to catalyze N-acetyl amino acids to enantiomerically pure L-amino acids .
Application
Acylase I from porcine kidney has been used to study the acylase I-catalyzed deacetylation of various S-alkyl-N-acetyl-L-cysteines and their carbon and oxygen analogues . Acylase I may be useful to catalyze N-acetyl amino acids to enantiomerically pure L-amino acids.
Biological Functions
Aminoacylases (N-acyl-L-amino acid amidohydrolases; EC 3.5.1.14) are widely found in animals, plants and microorganisms. The primary function of these enzymes is to remove acyl residues from N-acetylated amino acids although they may also be capable of hydrolysing carboxylic acid amides to fatty acid anions and L-amino acids. Although the catalytic mechanism of aminoacylases has been known for decades, the physiological role of these enzymes is still poorly understood. Activities of a similar nature, however, have been found in certain carboxypeptidases, aminopeptidases and dipeptidases. It could be therefore suggested that aminoacylases have a role to play in protein/peptide turnover.
General Description
Acylase I belongs to the aminoacylase family of enzymes.
Enzyme inhibitor
Aminoacylase is a metallo-enzyme that needs Zinc (Zn2+) as a cofactor to function. The Zinc ions inside of aminoacylase are each coordinated to histidine, glutamate, aspartate, and water. The Zinc ion polarizes the water, facilitating its deprotonation by a nearby basic residue. The negatively charged hydroxide ion is nucleophilic and attacks the electrophilic carbonyl carbon of the substrate's acyl group.The exact mechanism after this point is unknown, with one possibility being that the carbonyl then reforms, breaks the amide bond, and forms the two products. At some point in the mechanism, another water molecule enters and coordinates with Zinc, returning the enzyme to its original state.
The nucleophilic attack by water is the rate-limiting step of aminoacylase's catalytic mechanism. This nucleophilic attack is reversible while the subsequent steps are fast and irreversible. This reaction sequence is an example of Michaelis–Menten kinetics, allowing one to determine KM, Kcat, Vmax, turnover number, and substrate specificity through classic Michaelis-Menten enzyme experiments. The second and third forward steps cause the formation and release of the reaction's products.
Aminoacylase Preparation Products And Raw materials
Raw materials
Preparation Products
| Supplier | Tel | Country | ProdList | Advantage | Inquiry |
|---|---|---|---|---|---|
| Anthem Biosciences Pvt Ltd | +91-8066724000 +91-9900044575 | Karnataka, India | 38 | 58 | Inquiry |
| TCI Chemicals (India) Pvt. Ltd. | 1800 425 7889 | New Delhi, India | 6778 | 58 | Inquiry |
| Sisco Research Laboratories Pvt. Ltd. | +91-22-4268 5800 | Mumbai, India | 4317 | 58 | Inquiry |
| Triveni chemicals | 08048762458 | New Delhi, India | 6093 | 58 | Inquiry |
| career henan chemical co | +86-0371-86658258 +8613203830695 | China | 29897 | 58 | Inquiry |
| Chongqing Chemdad Co., Ltd | +86-023-6139-8061 +86-86-13650506873 | China | 39916 | 58 | Inquiry |
| Hefei TNJ Chemical Industry Co.,Ltd. | 0551-65418671 | China | 34571 | 58 | Inquiry |
| Shanghai UCHEM Inc. | +862156762820 +86-13564624040 | China | 7034 | 58 | Inquiry |
| Henan Alfa Chemical Co., Ltd | +8618339805032 | China | 13186 | 58 | Inquiry |
| Ningxia LabsChem Co., Ltd. | 0952-9294929 +8613895679378 | China | 352 | 58 | Inquiry |
9012-37-7(Aminoacylase)Related Search:
1of4
chevron_right




