Pravastatin
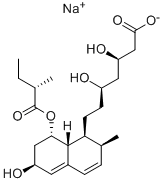
- CAS No.
- 81093-37-0
- Chemical Name:
- Pravastatin
- Synonyms
- MEVALOTIN;PRAVACHOL;ELISOR;KOPOSTAT;Pravatatin;PRAVASELECT;PRAVASTATIN;Pravastatin-D9;PRAVASTATIN(RG);EPTASTATIN SODIUM
- CBNumber:
- CB6317122
- Molecular Formula:
- C23H36O7
- Molecular Weight:
- 424.53
- MOL File:
- 81093-37-0.mol
- MSDS File:
- SDS
- Modify Date:
- 2024/5/8 14:48:35
| Melting point | 171.2-173 °C |
|---|---|
| Boiling point | 634.5±55.0 °C(Predicted) |
| Density | 1.21±0.1 g/cm3(Predicted) |
| storage temp. | Sealed in dry,Room Temperature |
| solubility | H2O: 19 mg/mL |
| pka | 4.31±0.10(Predicted) |
| form | powder |
| color | white |
| CAS DataBase Reference | 81093-37-0(CAS DataBase Reference) |
| EPA Substance Registry System | 1-Naphthaleneheptanoic acid, 1,2,6,7,8,8a-hexahydro-.beta.,.delta.,6-trihydroxy-2-methyl-8-[(2S)-2-methyl-1-oxobutoxy]-, (.beta.R,.delta,R,1S,2S,6S,8S,8aR)- (81093-37-0) |
SAFETY
Risk and Safety Statements
| Symbol(GHS) |   GHS08,GHS09 |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Signal word | Danger | |||||||||
| Hazard statements | H350-H360--H410 | |||||||||
| Precautionary statements | P273-P260-P314-P501 | |||||||||
| Hazard Codes | F,C | |||||||||
| Risk Statements | 11-34 | |||||||||
| Safety Statements | 16-26-36/37/39-45 | |||||||||
| WGK Germany | 2 | |||||||||
| RTECS | QJ7185000 | |||||||||
| NFPA 704 |
|
Pravastatin Chemical Properties,Uses,Production
Description
Pravastatin is the third HMG-CoA reductase inhibitor introduced for the treatment of atherosclerosis. Compared with lovastatin and simvastatin launched earlier, pravastatin is equipotent as an HMG-CoA reductase inhibitor in vitro, yet it is reported to be more tissue-selective.
Chemical Properties
Off-white Cryst
Uses
antiglaucoma,
General Description
Pravastatin, sodium 1,2,6,7,8,8a-hexahydro-β,δ,6-trihydroxy-2-methyl-8-(2-methyl-1-oxobutoxy)-1-naphthaleneheptanoate (Pravachol), is the most rapid actingof the three HMG-CoA reductase inhibitor drugs, reachinga peak concentration in about 1 hour. The sodium salt of theβ-hydroxy acid is more hydrophilic than the lactone forms ofthe other two agents, which may explain this property. In addition,the open form of the lactone ring contributes to a morehydrophilic agent, which, in turn, results in less CNS penetration.This explains, in part, why pravastatin has fewer CNSside effects than the more lipophilic lactone ester of this classof agents. Absorption of pravastatin following oral administrationcan be inhibited by resins such as cholestyramine becauseof the presence of the carboxylic acid function on thedrug. The lactone forms of lovastatin and simvastatin are lessaffected by cholestyramine.
Pravastatin Preparation Products And Raw materials
Raw materials
1of2
chevron_rightPreparation Products
| Supplier | Tel | Country | ProdList | Advantage | Inquiry |
|---|---|---|---|---|---|
| OCEAN TRADING CORPORATION | +91(22) 24921669 | New Delhi, India | 6211 | 58 | Inquiry |
| Medilink Pharmachem | +91 (79) 3007-0133 | New Delhi, India | 424 | 50 | Inquiry |
| Triveni chemicals | 08048762458 | New Delhi, India | 6093 | 58 | Inquiry |
| SynZeal Research Pvt Ltd | +1 226-802-2078 | Gujarat, India | 6522 | 58 | Inquiry |
| Pharmaffiliates Analytics and Synthetics P. Ltd | +91-172-5066494 | Haryana, India | 6773 | 58 | Inquiry |
| First Continental Online Pharmacy | 09910470260 | Kolkata, India | 2 | 58 | Inquiry |
| Koshambh Multitred Private Limited | 08048250245Ext 483 | Vadodara, India | 3 | 58 | Inquiry |
| Vista Inc. | 09177778907 | Maharashtra, India | 7 | 58 | Inquiry |
| CONCORD BIOTECH LIMITED | +91 79 68138700 | New Delhi, India | 11 | 58 | Inquiry |
| Luna Chemical Industries Private Limited | 08048372754Ext 585 | Vadodara, India | 22 | 58 | Inquiry |
81093-37-0(Pravastatin)Related Search:
1of4
chevron_right




