Zeolite
- CAS No.
- 1318-02-1
- Chemical Name:
- Zeolite
- Synonyms
- Zeolites;Aluminosilicate;SODIUM ALUMINOSILICATE;radiolite;MOLECULAR SIEVE 4A;MOLECULAR SIEVES 3A;SODIUM ALUMINUM SILICATE;MOLECULAR SIEVES 4A;SODIUM ALUMINIUM SILICATE;SGK
- CBNumber:
- CB6330194
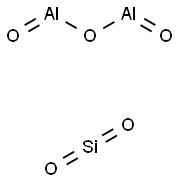
- Molecular Formula:
- Al2O5Si
- Molecular Weight:
- 162.045576
- MOL File:
- 1318-02-1.mol
- MSDS File:
- SDS
- Modify Date:
- 2024/9/4 23:37:13
| Melting point | >1600°C |
|---|---|
| Density | 1.9~2.3g/cm3 |
| storage temp. | no restrictions. |
| form | powder |
| pka | 10.348[at 20 ℃] |
| color | White |
| PH | 8-11 (H2O) |
| Odor | wh., odorless powd. |
| Water Solubility | Insoluble in water. |
| Sensitive | Hygroscopic |
| Exposure limits |
ACGIH: TWA 10 mg/m3 OSHA: TWA 15 mg/m3 NIOSH: IDLH 5000 mg/m3; TWA 2.4 mg/m3; TWA 0.3 mg/m3 |
| IARC | 3 (Vol. 68) 1997 |
| EPA Substance Registry System | Zeolites (1318-02-1) |
SAFETY
Risk and Safety Statements
| Hazard Codes | Xn | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Risk Statements | 36/37/38-36/37-20 | |||||||||
| Safety Statements | 26-7/9-36-22 | |||||||||
| WGK Germany | 3 | |||||||||
| RTECS | ZG6800000 | |||||||||
| F | 3 | |||||||||
| HS Code | 2842 10 00 | |||||||||
| NFPA 704 |
|
Zeolite price More Price(81)
| Manufacturer | Product number | Product description | CAS number | Packaging | Price | Updated | Buy |
|---|---|---|---|---|---|---|---|
| Sigma-Aldrich(India) | 96096 | Zeolite | 1318-02-1 | 100G | ₹8432.68 | 2022-06-14 | Buy |
| Sigma-Aldrich(India) | 96096 | Zeolite | 1318-02-1 | 500G | ₹24518.63 | 2022-06-14 | Buy |
| Sigma-Aldrich(India) | 643653 | Aluminosilicate, mesostructured MCM-41 (hexagonal) | 1318-02-1 | 5G | ₹17601.45 | 2022-06-14 | Buy |
| Sigma-Aldrich(India) | 643653 | Aluminosilicate, mesostructured MCM-41 (hexagonal) | 1318-02-1 | 25G | ₹69182.58 | 2022-06-14 | Buy |
| Sigma-Aldrich(India) | 334448 | Molecular sieves powder, Catalyst support, sodium Y zeolite | 1318-02-1 | 100G | ₹7934.73 | 2022-06-14 | Buy |
Zeolite Chemical Properties,Uses,Production
Occurrence
Aluminosilicates are obtained directly from the aluminosilicate rocks or as synthetic materials. These oxides have the general formula: mAl2O3 . nSiO, e.g., andalusite, sillimanite, disthene, kaolinite, topaz, and mullite. Natural aluminosilicate powders used in investment casting are supplied with various Al2O3 content, such as 42–47% (e.g., Molochite) and 60% (e.g., Remasil, Mulgrain), and with gradation in the range of 325# 120#. The higher the content of Al2O3 in the powder, the higher its resistance to temperature. Most common contaminants in aluminosilicate powder are: Fe2O3, TiO2, and ZrO2, while the content of CaO, MgO, K2O, P2O5, and Cr2O3 is very small. An important advantage of aluminosilicate powders is their availability and low price of about 0.5 €/kg.
Mullite 3Al2O3 . 2SiO2 is a mineral that is rarely found in nature (known deposits on the Isle of Mull, Scotland). For industrial purposes, synthetic mullite is used almost exclusively (sintered or melted) with particle sizes up to: 0.07, 0.12, 0.25–0.5, 0.5–1.0 mm and of white color. The raw material is milled and fractionated for a suitable grain size matrix of the ceramic powders and stucco. The melting point of mullite is 1810 ℃ and density is 3.05 g/cm3, which does not depend on the temperature. It is characterized by high resistance to creep and thermal shock due to a low coefficient of thermal expansion and a low thermal conductivity. Synthetic mullite is characterized by a high chemical purity—total content of impurities does not exceed 0.5 wt%. The price is high (about 2.5 V/kg), thus it is used for ceramic molds for casting turbine blades with a DS or SC microstructure.
Uses
Zeolite is used in formation of Silicates.
Preparation
Zeolite family is a well-known class of alkali aluminosilicates found in nature that has been used as ion exchange minerals in water softening.They are hydrated systems and give up part of their water molecules quite readily at modest temperatures but reabsorb the moisture when exposed to moist air. A similar alkali aluminosilicate is prepared artificially and has the empirical composition 2M2O·Al203·3SiO2·3H20; these compositions are called "permutites" and are usually prepared with sodium as the alkali metal by adding aluminum sulphate to soluble silicate solutions or by combining sodium aluminate with sodium silicate. In the presence of calcium, iron and other cations, the sodium ion is displaced by the heavier metal ions and the permutites can thus be used to remove these metal ions from solution—for example, to remove hardness from hard water, or to "soften" water. The permutite or artificial zeolite can be regenerated by treating with strong sodium chloride solution, thus replacing the calcium or iron by sodium. More recently, the use of synthetic zeolites as "molecular sieves" has become quite important in the separation of organic compounds or as catalysts in petroleum refining. In these alkali aluminosilicates, the pore size is determined by the alkali metal cation used.
Definition
A natural hydrated silicate of aluminum and either sodium or calcium or both, of the type Na2O?Al2O3?xSiO2?xH2O. Both natural and artificial zeolites are used extensively for water softening, as detergent builders, and cracking catalysts. For the former purpose the sodium or potassium compounds are required, since their usefulness depends on the cationic exchange of the sodium of the zeolite for the calcium or magnesium of hard water. When the zeolite has become saturated with calcium or magnesium ion it is flooded with strong salt solution, a reverse exchange of cations takes place and the material is regenerated. The natural zeolites are analcite, chabazite, heulandite, natrolite, stilbite, and thomsonite. Synthetic zeolites are made either by a gel process (sodium silicate and alumina) or by a clay process (kaolin), which forms a matrix to which the zeolite is added. These processes are quite complex, involving substitution of various rare-earth oxides. The effectiveness of zeolites depends on their pore size, which may be as small as 4–5 A? . Other applications of zeolites are as adsorbents, desiccants, and in solar collectors, where they function as both heating and cooling agent.
General Description
Aluminosilicate, mesostructured (Al-MSU) is an analog of hexagonal MCM-41 that can be assembled by zeolite seeds. It is hydrothermally stable and strongly acidic, which makes it useful in catalysis and adsorption technology.
Agricultural Uses
Zeolite is a generic term used for any of the large group of
minerals, comprising hydrated aluminosilicates of Na,
K, Ca and Ba. These can readily be dehydrated and rehydrated,
and find application as molecular sieves and
cation exchangers.
Natural zeolites are analcite, chabazite, heulandite,
natrolite, stilbite and thomsonite. Artificial zeolites are
made in many forms (ranging from gelatinous, to porous,
to sand like varieties) and are used as gas absorbents,
drying agents, catalysts and water softeners. Zeolites
include a diverse group of compounds of sulphonated
organic or basic resins.
Zeolites are low-temperature and low-pressure
minerals occurring commonly in basalts, denitrification
products and as alteration products of feldspars and
nepheline.
Zeolites are also present in volcanic ash clay
minerals. When applied to land or similar fields, they
have a strong capacity for holding ammonium ions which
reduce the leaching of the nitrate ions.
Zeolites are called molecular sieves because of their
use in separating mixtures by selective absorption. They
are also used in sorption pumps for vacuum systems
(e.g., In permutil), ion exchange systems (for water
softening) and as catalysts.
Zeolite Preparation Products And Raw materials
Raw materials
Preparation Products
| Supplier | Tel | Country | ProdList | Advantage | Inquiry |
|---|---|---|---|---|---|
| GLR Innovations | +91 9891111994 | New Delhi, India | 4535 | 58 | Inquiry |
| Otto Chemie Pvt Ltd | +91-2222070099 +91-2266382599 | Maharashtra, India | 429 | 58 | Inquiry |
| Process Enterprises | +91-9752326822 +91-9827225090 | Madhya Pradesh, India | 5 | 58 | Inquiry |
| Manek Minerals | +91-2832240199 +91-2832240699 | Gujarat, India | 10 | 58 | Inquiry |
| Pallav Chemicals And Solvents Pvt Ltd | +91-9136093115 +91-9136093115 | Maharashtra, India | 1364 | 58 | Inquiry |
| ARRAKIS INDUSTRIES LLP | +91 74995 32711 | Maharashtra, India | 1353 | 58 | Inquiry |
| Nanochemazone Inc. | 08048616195 | Haryana, India | 79 | 58 | Inquiry |
| Process Enterprises | 91-731-2877129 | Madhya Pradesh, India | 4 | 58 | Inquiry |
| Monarch International | 91-11-43770000 | Delhi, India | 8 | 58 | Inquiry |
| Astrra Chemicals | 91-44-28273947 | Tamil Nadu, India | 32 | 58 | Inquiry |
| Supplier | Advantage |
|---|---|
| GLR Innovations | 58 |
| Otto Chemie Pvt Ltd | 58 |
| Process Enterprises | 58 |
| Manek Minerals | 58 |
| Pallav Chemicals And Solvents Pvt Ltd | 58 |
| ARRAKIS INDUSTRIES LLP | 58 |
| Nanochemazone Inc. | 58 |
| Process Enterprises | 58 |
| Monarch International | 58 |
| Astrra Chemicals | 58 |
Related articles
- Zeolite: The Versatile Material Shaping Modern Chemistry
- Zeolites, a group of unique and naturally occurring minerals, are aluminosilicate compounds characterized by their stable and ....
- May 15,2024
- Titanium Silicalite-1 - TS-1 Catalyst
- Titanium silicalite-1 is widely used in industry owing to its ability to catalytically epoxidize olefins with hydrogen peroxid....
- Mar 16,2023
- Characteristic and Uses of Titanium Silicalite-1
- Titanium Silicalite-1, TS-1, was the first framework-substituted redox molecular sieve to be identified. Redox molecular sieve....
- Jun 13,2022
1318-02-1(Zeolite)Related Search:
1of4
chevron_right




