URANIUM(VI) OXIDE
- CAS No.
- 1344-58-7
- Chemical Name:
- URANIUM(VI) OXIDE
- Synonyms
- UO3;trioxouranium;triketouranium;URANIUM TRIOXIDE;URANIUM(VI) OXIDE;Uranium oxide (UO3);URANIUM OXIDE YELLOW;Uranyl oxide, orange;Uranium(VI) oxide, red;URANIUM (VI) OXIDE 99.8%
- CBNumber:
- CB6362445
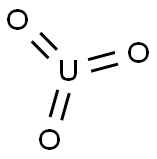
- Molecular Formula:
- O3U
- Molecular Weight:
- 286.03
- MOL File:
- 1344-58-7.mol
- Modify Date:
- 2024/8/28 13:53:25
| Melting point | °Cd ec.) | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Density | 7,29 g/cm3 | ||||||||||||||
| solubility | insoluble in H2O; soluble in acid solutions | ||||||||||||||
| form | orange-yellow crystals | ||||||||||||||
| color | orange-yellow crystals, crystalline | ||||||||||||||
| Water Solubility | insoluble H2O; soluble acids [MER06] | ||||||||||||||
| crystal system | Nogata | ||||||||||||||
| Space group | Fddd | ||||||||||||||
| Lattice constant |
|
||||||||||||||
| EPA Substance Registry System | Uranium trioxide (1344-58-7) |
URANIUM(VI) OXIDE Chemical Properties,Uses,Production
Chemical Properties
-100 mesh; has six forms: α is hexagonal brown, β is orange monoclinic, γ is bright yellow rhomb, δ is red cub, ε is brick red tricl, ηis rhomb; UO3 can be obtained by thermal decomposition of uranyl compounds, e.g, carbonates, oxalates nitrates [KIR83] [CER91]
Uses
Uranium trioxide, UO3, is a versatile solid that has important applications in the nuclear fuel cycle.
Synthesis
Pure UO3 is difficult to prepare because the thermal cleavage of uranyl compounds does not free the product of traces of volatile components, while at high temperatures dissociation into U3O8 and O2 becomes objectionable. To circumvent these drawbacks it is desirable to use O2 at a pressure above atmospheric.
A weighing tube is charged with 5-10 g. of the dry peroxide and placed (unstoppered) in an electric crucible furnace preheated to 350°C. A fast stream of O2 is admitted through the opening in the furnace lid. The temperature is initially held for 3-5 hours at 350°C and then for one half to one hour at 400°C. The weighing tube is then stoppered and allowed to cool in a desiccator.
Purification Methods
The oxide is dissolved in HClO4 (to give a uranium content of 5%), and the solution is adjusted to pH 2 by addition of dilute ammonia. Dropwise addition of 30% H2O2, with rapid stirring, precipitated U(VI) peroxide, the pH being held constant during the precipitation, by addition of small amounts of the ammonia solution. Then H2O2 is added until further quantities caused no change in pH. After stirring for 1hour, the slurry is filtered through coarse filter paper in a Büchner funnel, washed with 1% H2O2 acidified to pH 2 with HClO4, then heated at 350o for three days in a large platinum dish [Baes J Phys Chem 60 878 1956].
URANIUM(VI) OXIDE Preparation Products And Raw materials
| Supplier | Tel | Country | ProdList | Advantage | Inquiry |
|---|---|---|---|---|---|
| Otto Chemie Pvt. Ltd. | +91 9820041841 | Mumbai, India | 5873 | 58 | Inquiry |
| ShanDong Look Chemical Co.,Ltd. | +8617653113219 | China | 2739 | 58 | Inquiry |
| Shaanxi Didu New Materials Co. Ltd | +86-89586680 +86-13289823923 | China | 8673 | 58 | Inquiry |
| Guangdong Fangxin Biotechnology Co., Ltd. | 0751-2838688 13376594225 | China | 9939 | 58 | Inquiry |
| Henan Lien Chemical Products Co., Ltd. | 15378766539 | China | 3968 | 58 | Inquiry |
| Hubei Junrui Biotechnology Co., Ltd | 13971166412 | China | 4375 | 58 | Inquiry |
| MATERION Inc. | 800 327 1355 | United States | 1079 | 74 | Inquiry |
| Pfaltz & Bauer, Inc. | (800) 225-5172 | United States | 6830 | 72 | Inquiry |
| MV Laboratories, Inc. | 908 996 6633 | United States | 581 | 42 | Inquiry |
| Service Chemical Inc. | 888-895-6920 | Germany | 6373 | 71 | Inquiry |
1344-58-7(URANIUM(VI) OXIDE)Related Search:
1of2
chevron_right




