B581
- CAS No.
- 149759-96-6
- Chemical Name:
- B581
- Synonyms
- FTASE INHIBITOR I;B581;B581 USP/EP/BP;H-Cys-()-Val-()-Phe-Met-OH;CYS-((R))-VAL-((R))-PHE-MET;H-CYS-(R)-VAL-(R)-PHE-MET-OH;H-Cys-psi(CH2NH)Val-psi(CH2NH)Phe-Met-OH;N-[(S)-2-((R)-2-Amino-3-mercapto-propylamino)-3-me;H-Cys-psi(CH2NH)Val-psi(CH2NH)Phe-Met-OH trifluoroacetate salt;N-[2(S)-[2(R)-AMINO-3-MERCAPTOPROPYLAMINO]-3-METHYL-BUTYL]-PHE-MET
- CBNumber:
- CB6454713
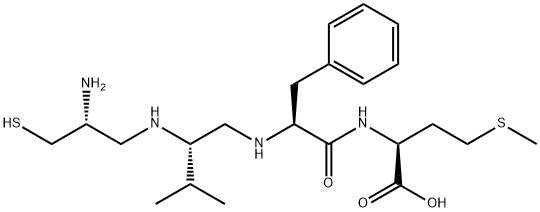
- Molecular Formula:
- C22H38N4O3S2
- Molecular Weight:
- 470.69
- MOL File:
- 149759-96-6.mol
- MSDS File:
- SDS
- Modify Date:
- 2023/5/18 11:31:20
B581 Chemical Properties,Uses,Production
Description
FTase inhibitor I is a potent inhibitor of farnesyltransferase (FTase; IC50 = 21 nM) that is greater than 30-fold selective for FTase over geranylgeranyl transferase (GGTase; IC50 = 790 nM). It prevents farnesylation of Ras and inhibits proliferation of cells transformed by Ras farnesylation. In a dog model of subarachnoid hemorrhage, it reduces GTP-Ras in spastic basilar arteries, decreases angiographic vasospasm, and improves clinical scores.
Uses
FTase Inhibitor I is a potent, selective, peptidomimetic FTase inhibitor.
Definition
ChEBI: A dipeptide obtained from the tetrapeptide Cys-Val-Phe-Met by reduction of the amide carbonyl groups of the Cys and Val residues.
Enzyme inhibitor
This cell-permeable CAAX peptidomimetic (FW = 470.70 g/mol; FWtrifluoracetate-salt = 583.71 g/mol; CAS 149759-96-6), named as N-[2(S)- (2(R)-2-amino-3-mercaptopropylamino)-3-methylbutyl]-L-phenylalanyl-L methionine), binds to and prevents protein farnesyltransferase (IC50 = 21 nM) from interacting with C-terminal L-Cys-L-Val-L-Phe-L-Met substrate recognition sequences that are present in its natural substrates. Otherwise hydrophilic proteins associate with membranes by means of enzymatic attachment of hydrophobic moieties to their C-termini. Whereas prenylation occurs in the cytosol, post-prenylation processing is accomplished on the cytoplasmic surface of the endoplasmic reticulum and Golgi apparatus. B581 inhibits prenylation and processing of H-ras and lamin A. B581 specifically blocks farnesylated, but not geranylgeranylated or myristylated, oncogenic ras signaling and transformation.
B581 Preparation Products And Raw materials
Raw materials
Preparation Products
| Supplier | Tel | Country | ProdList | Advantage | Inquiry |
|---|---|---|---|---|---|
| CLEARSYNTH LABS LTD. | +91-22-45045900 | Hyderabad, India | 6351 | 58 | Inquiry |
| Alpha Biopharmaceuticals Co., Ltd | +86-15542445688 | China | 888 | 58 | Inquiry |
| CONIER CHEM AND PHARMA LIMITED | +8618523575427 | China | 49391 | 58 | Inquiry |
| TargetMol Chemicals Inc. | +1-781-999-5354 +1-00000000000 | United States | 19892 | 58 | Inquiry |
| Dideu Industries Group Limited | +86-29-89586680 +86-15129568250 | China | 26165 | 58 | Inquiry |
| Nextpeptide Inc | +86-0571-81612335 +8613336028439 | China | 19915 | 58 | Inquiry |
| Nanjing TGpeptide | +86-13347807150 +86-13347807150 | China | 3279 | 58 | Inquiry |
| Aladdin Scientific | +1-+1(833)-552-7181 | United States | 52927 | 58 | Inquiry |
| Nantong Hanfang Biotechnology Co. , Ltd. | 18616537568 | China | 30968 | 58 | Inquiry |
| Hangzhou Peptidego Biotech Co.,Ltd. | 0571-87213919 | China | 6526 | 58 | Inquiry |
149759-96-6(B581)Related Search:
1of4
chevron_right




