Silver azide
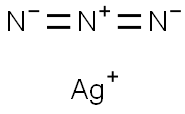
- CAS No.
- 13863-88-2
- Chemical Name:
- Silver azide
- Synonyms
- Silver azide;Silver(1+)azide;Silver(I) azide;Silver azide (Ag(N3))
- CBNumber:
- CB6852078
- Molecular Formula:
- AgN3
- Molecular Weight:
- 149.8883
- MOL File:
- 13863-88-2.mol
- MSDS File:
- SDS
- Modify Date:
- 2023/10/17 17:11:50
| Melting point | 212°C |
|---|---|
| Density | 4.350 |
| form | colorless rhombohedral crystals |
| color | orthorhombic crystals, crystalline; explodes, explosive |
| Water Solubility | 0.01g/100mL H2O (100°C) [CRC10] |
| Solubility Product Constant (Ksp) | pKsp: 8.54 |
| EPA Substance Registry System | Silver azide (13863-88-2) |
SAFETY
Risk and Safety Statements
| Symbol(GHS) |  GHS01 |
|---|---|
| Signal word | Danger |
| Hazard statements | H200 |
| Precautionary statements | P201-P202-P281-P372-P373-P380-P401-P501 |
Silver azide Chemical Properties,Uses,Production
Chemical Properties
orthorhombic, a=0.559 nm, b=0.591 nm, c=0.610nm; can be prepared by reacting silver nitrate solution with hydrazine or hydrazoic acid; sensitive to shock [KIR83] [CIC73]
Uses
It is used as a primary explosive, often as areplacement for lead azide. It may be usedin smaller quantities than lead azide as ainitiator.
Health Hazard
Silver azide is a highly toxic substance. Thetoxic effects have not been reported.
Fire Hazard
It is a primary explosive. It explodes violently
upon thermal and mechanical shock.
It requires lesser energy for initiation than
lead azide and also fires with a shorter time
delay. The heats of combustion and detonation
are 1037 and 454 cal/g, respectively
(i.e., 156 and 68 kcal/mol, respectively). The
detonation velocity is 6.8 km/sec (at the crystal
density 5.1 g/cm3). The pure compound
explodes at 340°C (644°F) (Mellor 1967).
The detonation can occur at much lower temperatures
in an electric field when initiated
by irradiation. Also, the presence of impurities
can lower down the temperature of
detonation. Such impurities include oxides,
sulfides, and selenides of copper and other
metals.
It reacts with halogens forming halogen
azides, often producing explosions. It reacts
explosively with chlorine dioxide, most interhalogen
compounds, and many dyes. It reacts
with acids forming heat- and shock-sensitive
hydrazoic acid. When mixed with salt solutions
of heavy metals, their azides are
formed, which are sensitive to heat, impact
and electric charge.
Safety Profile
Explodes when heated above 270°C or on impact. Pure silver azide explodes at 340℃. An electric field or irradiation by electron pulses can explode the crystals. Shock-sensitive when dry and has detonated @ 250℃. Solutions in aqueous ammonia explode above 100°C. Reacts to form more explosive products with iodine (forms iodine azide); bromine and other halogens. The presence of metal oxides or metal sulfides increases the azide's sensitivity to explosion. lMixtures with sulfur dioxide are explosive. When heated to decomposition it emits toxic fumes of NOx. See also AZIDES and SILVER COMPOUNDS.
Silver azide Preparation Products And Raw materials
| Supplier | Tel | Country | ProdList | Advantage | Inquiry |
|---|---|---|---|---|---|
| AFINE CHEMICALS LIMITED | +86-0571-85134551 | China | 15395 | 58 | Inquiry |
| Shaanxi Didu New Materials Co. Ltd | +86-89586680 +86-13289823923 | China | 8673 | 58 | Inquiry |
| GIHI CHEMICALS CO.,LIMITED | +8618058761490 | China | 50000 | 58 | Inquiry |
| Hubei Enxing Biotechnology Co., Ltd | 16621771607 | China | 8253 | 58 | Inquiry |
| Supplier | Advantage |
|---|---|
| AFINE CHEMICALS LIMITED | 58 |
| Shaanxi Didu New Materials Co. Ltd | 58 |
| GIHI CHEMICALS CO.,LIMITED | 58 |
| Hubei Enxing Biotechnology Co., Ltd | 58 |
13863-88-2(Silver azide)Related Search:
1of2
chevron_right




