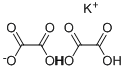
potassium trihydrogen dioxalate
- CAS No.
- 127-96-8
- Chemical Name:
- potassium trihydrogen dioxalate
- Synonyms
- Einecs 204-874-6;Tetraoxalate potassium;Potassium Oxalate, >98%;PotassiumTetraoxalateA.R.;POTASSIUMTETRAOXALATE,REAGENT;PotassiumOxalateK2C2O4*H2O>98%;potassium trihydrogen dioxalate;Oxalic acid potassium salt (2:1);OxalicAcidPotassiumSaltDihydrate99%;Ethanedioic acid, potassium salt (2:1)
- CBNumber:
- CB6932051
- Molecular Formula:
- C2H4K2O6
- Molecular Weight:
- 202.24616
- MOL File:
- 127-96-8.mol
- MSDS File:
- SDS
- Modify Date:
- 2023/5/4 15:17:01
| Melting point | decomposes [CRC10] |
|---|---|
| Density | 1.875[at 20℃] |
| vapor pressure | 0Pa at 20℃ |
| Water Solubility | soluble 60 parts cold H2O, 12 parts boiling H2O; slightly soluble alcohol [MER06] |
| EPA Substance Registry System | Ethanedioic acid, potassium salt (2:1) (127-96-8) |
SAFETY
Risk and Safety Statements
| Symbol(GHS) |   GHS07,GHS05 |
|---|---|
| Signal word | Danger |
| Hazard statements | H312-H302 |
| Precautionary statements | P280-P302+P352-P312-P322-P363-P501-P264-P270-P301+P312-P330-P501 |
potassium trihydrogen dioxalate Chemical Properties,Uses,Production
Chemical Properties
colorless or white crystal(s) [MER06]
Physical properties
The monohydrate is a white crystalline solid; monoclinic structure; density 2.13 g/cm3; loses its water at about 160°C; converts to carbonate when ignited; effloresces in warm dry air; soluble in water, 33 g/100 mL at 20°C; a 0.05m solution of K2C2O4?2H2O has a pH 1.679.
Occurrence
Potassium oxalate, along with calcium oxalate, is found in leaves and roots of certain plants. It is used for cleaning and bleaching straw and for removing stains. It also is used in photography, in clinical tests, as a secondary pH standard, and in wet chemical analysis. The analytical application involves standardization of many oxidizing agents in titrimetric analysis.
Preparation
Potassium oxalate can be preparaed by heating potassium formate at 360°C:
2HCOOK →K2C2O4+ H2
The salt is obtained as its monohydrate by neutralization of oxalic acid with a dilute aqueous solution of potassium hydroxide followed by crystallization:
H2C2O4+ 2KOH →K2C2O4+ 2H2O.
Purification Methods
Crystallise it from water below 50o. Dry it below 60o at 760mm. [See potassium oxalate above, Beilstein 2 IV 1823.]
potassium trihydrogen dioxalate Preparation Products And Raw materials
Raw materials
Preparation Products
potassium trihydrogen dioxalate Suppliers
| Supplier | Tel | Country | ProdList | Advantage | Inquiry |
|---|---|---|---|---|---|
| New Alliance Dye Chem Pvt Ltd | +91-9820112338 +91-9820112338 | Maharashtra, India | 104 | 58 | Inquiry |
| SHANDONG ZHI SHANG CHEMICAL CO.LTD | +86 18953170293 | China | 2931 | 58 | Inquiry |
| SIMAGCHEM CORP | +86-13806087780 | China | 17367 | 58 | Inquiry |
| Hefei TNJ Chemical Industry Co.,Ltd. | 0551-65418671 | China | 34571 | 58 | Inquiry |
| Hebei Guanlang Biotechnology Co,.LTD | +86-19930503253; +8619930503252 | China | 5910 | 58 | Inquiry |
| LEAPCHEM CO., LTD. | +86-852-30606658 | China | 43348 | 58 | Inquiry |
| Shanghai Likang New Materials Co., Limited | +86-16631818819 +86-17736933208 | China | 9311 | 58 | Inquiry |
| JYS | 15307147230 | China | 1772 | 58 | Inquiry |
| Condice Chemical (Hubei) Co., Ltd. | 18064039730 | China | 2831 | 58 | Inquiry |
| Langfang Ganyao Technology Co., LTD | 0316-2617888 17301291189 | China | 374 | 58 | Inquiry |
127-96-8(potassium trihydrogen dioxalate )Related Search:
1of3
chevron_right




