Potassium oxalate
- CAS No.
- 583-52-8
- Chemical Name:
- Potassium oxalate
- Synonyms
- Potassium oxalate;DIPTASSIUMOXALATE;ipotassium,oxalate;DI-POTASSIUM OXALATE;dipotassiumethanedioate;potassiumneutraloxalate;Oxalic acid dipotassium;potassiumoxalate(k2c2o4);oxalicacid,dipotassiumsalt;Potassium Oxalate Anhydrous
- CBNumber:
- CB9162721
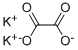
- Molecular Formula:
- C2K2O4
- Molecular Weight:
- 166.22
- MOL File:
- 583-52-8.mol
- MSDS File:
- SDS
- Modify Date:
- 2024/5/30 16:33:02
| Density | 2.13 |
|---|---|
| solubility | slightly soluble in H2O |
| color | white pwd |
| Odor | odorless |
| Water Solubility | 392g/L at 20℃ |
| CAS DataBase Reference | 583-52-8(CAS DataBase Reference) |
| EPA Substance Registry System | Ethanedioic acid, potassium salt (1:2) (583-52-8) |
SAFETY
Risk and Safety Statements
| Symbol(GHS) |  GHS07 |
|---|---|
| Signal word | Warning |
| Hazard statements | H312-H302-H319 |
| Precautionary statements | P264-P270-P301+P312-P330-P501-P280-P302+P352-P312-P322-P363-P501-P264-P280-P305+P351+P338-P337+P313P |
Potassium oxalate Chemical Properties,Uses,Production
Chemical Properties
Colorless, transparent crystals; odorless. Soluble in water.
Uses
Potassium oxalate is a white crystal or powder made by neutralizing oxalic acid with potassium carbonate. It is soluble in water 1:3 but not in alcohol. Potassium oxalate was used as an early developer for gelatin plates but is best known as the developer for platinum prints.
General Description
Potassium oxalate, K2C204, H20, is odorless, efforescent, water-soluble, colorless crystals that decompose when heated. Sinks in and mixes slowly with water. Used in analytical chemistry and photography, and as a bleach and oxalic acid source.
Air & Water Reactions
Water soluble.
Reactivity Profile
Potassium oxalate gives basic aqueous solutions. Reacts as a base to neutralize acids in reactions that generate heat, but less than is generated by neutralization of the bases in reactivity group 10. Can serve as a reducing agent in reactions that generate carbon dioxide.
Hazard
Toxic by inhalation and ingestion.
Health Hazard
Inhalation of dust can cause systemic poisoning. Ingestion causes burning pain in throat, esophagus, and stomach; exposed areas of mucous membrane turn white; vomiting, severe purging, weak pulse, and cardiovascular collapse may result; if death is delayed, neuromuscular symptoms develop. Contact with eyes or skin causes irritation.
Fire Hazard
Behavior in Fire: Loses water at about 160° and decomposes to carbonate with no charring. The reaction is not hazardous.
Potassium oxalate Preparation Products And Raw materials
Raw materials
Preparation Products
| Supplier | Tel | Country | ProdList | Advantage | Inquiry |
|---|---|---|---|---|---|
| Indiana ChemPort | +91-91-2652638667 +91-7574886711 | Gujarat, India | 85 | 58 | Inquiry |
| New Alliance Dye Chem Pvt Ltd | +91-9820112338 +91-9820112338 | Maharashtra, India | 104 | 58 | Inquiry |
| Nithyasri Chemicals (APURVA CHEMICALS) | +91-2512692130 +91-9323652199 | Maharashtra, India | 75 | 58 | Inquiry |
| Triveni chemicals | 08048762458 | New Delhi, India | 6093 | 58 | Inquiry |
| Zama Chemical | 08048983954 | Mumbai, India | 217 | 58 | Inquiry |
| Vora Brothers | 08048950231 | Mumbai, India | 42 | 58 | Inquiry |
| Powder Pack Chem | 08046047024 | Mumbai, India | 191 | 58 | Inquiry |
| Vishnupriya Chemicals Private Limited | 08046041737 | Hyderabad, India | 220 | 58 | Inquiry |
| Nithyasri Chemicals | 08046044427 | Maharashtra, India | 43 | 58 | Inquiry |
| Orientallabs Retail Services Private Limited | 07942550683 | Kolkata, India | 3 | 58 | Inquiry |





