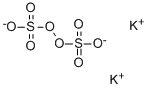
Potassium persulfate
- CAS No.
- 7727-21-1
- Chemical Name:
- Potassium persulfate
- Synonyms
- KPS;POTASSIUM PEROXODISULFATE;POTASSIUM PEROXYDISULFATE;POTASSIUM PERSULPHATE;PEROXYDISULFURIC ACID;Potassum Suphates;DIPOTASSIUM PEROXYDISULFATE;anthion;Virkon S;BETZ 2701
- CBNumber:
- CB6854294
- Molecular Formula:
- K2O8S2
- Molecular Weight:
- 270.32
- MOL File:
- 7727-21-1.mol
- MSDS File:
- SDS
- Modify Date:
- 2023/6/28 13:45:06
| Melting point | 1067 °C |
|---|---|
| Boiling point | 1689 °C |
| Density | 2.47 |
| vapor density | 9.3 (vs air) |
| vapor pressure | 0Pa at 25℃ |
| storage temp. | Store at +5°C to +30°C. |
| solubility | H2O: 0.5 M at 20 °C, clear, colorless |
| form | Solid |
| Specific Gravity | 2.477 |
| color | White |
| PH | 3.2 (50g/l, H2O, 20℃) |
| Odor | Odorless |
| PH Range | 2.5 - 4.5 |
| Water Solubility | 5 g/100 mL (20 ºC) |
| Merck | 14,7656 |
| Exposure limits | ACGIH: TWA 0.1 mg/m3 |
| Stability | Stable. Strong oxidizer. Incompatible with strong reducing agents, organic materials, combustible materials. |
| InChIKey | USHAGKDGDHPEEY-UHFFFAOYSA-L |
| LogP | -1 at 20℃ |
| CAS DataBase Reference | 7727-21-1(CAS DataBase Reference) |
| EPA Substance Registry System | Potassium persulfate (7727-21-1) |
SAFETY
Risk and Safety Statements
| Symbol(GHS) |    GHS03,GHS07,GHS08 |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Signal word | Danger | |||||||||
| Hazard statements | H272-H302-H315-H317-H319-H334-H335 | |||||||||
| Precautionary statements | P210-P280-P301+P312-P302+P352-P304+P340+P312-P305+P351+P338 | |||||||||
| Hazard Codes | O,Xn | |||||||||
| Risk Statements | 8-22-36/37/38-42/43 | |||||||||
| Safety Statements | 22-24-26-37 | |||||||||
| RIDADR | UN 1492 5.1/PG 3 | |||||||||
| WGK Germany | 1 | |||||||||
| RTECS | TT5900000 | |||||||||
| TSCA | Yes | |||||||||
| HS Code | 2833 40 00 | |||||||||
| HazardClass | 5.1 | |||||||||
| PackingGroup | III | |||||||||
| Toxicity | LD50 orally in Rabbit: 802 mg/kg | |||||||||
| NFPA 704 |
|
Potassium persulfate price More Price(23)
| Manufacturer | Product number | Product description | CAS number | Packaging | Price | Updated | Buy |
|---|---|---|---|---|---|---|---|
| Sigma-Aldrich(India) | 60489 | Potassium peroxodisulfate puriss. p.a., ACS reagent, ≥99.0% (RT) | 7727-21-1 | 250G | ₹3204.2 | 2022-06-14 | Buy |
| Sigma-Aldrich(India) | 60489 | Potassium peroxodisulfate puriss. p.a., ACS reagent, ≥99.0% (RT) | 7727-21-1 | 1KG | ₹8172.88 | 2022-06-14 | Buy |
| Sigma-Aldrich(India) | 379824 | Potassium persulfate 99.99% trace metals basis | 7727-21-1 | 5G | ₹6679.03 | 2022-06-14 | Buy |
| Sigma-Aldrich(India) | 379824 | Potassium persulfate 99.99% trace metals basis | 7727-21-1 | 25G | ₹25503.7 | 2022-06-14 | Buy |
| Sigma-Aldrich(India) | 216224 | Potassium persulfate ACS reagent, ≥99.0% | 7727-21-1 | 5G | ₹4059.38 | 2022-06-14 | Buy |
Potassium persulfate Chemical Properties,Uses,Production
Description
Potassium persulfate, is composed of white crystals that are soluble in water, and it decomposes below 212°F (100°C). Potassium persulfate is a dangerous fire risk in contact with organic materials. It is a strong oxidizing agent and an irritant, with a four-digit UN identification number of 1492. The primary uses are in bleaching, as an oxidizing agent, as an antiseptic, as a polymerization promoter, and in the manufacture of pharmaceuticals.
Chemical Properties
Potassium persulfate is a colorless or white, odorless crystalline material.
Physical properties
Colorless or white crystals; triclinic structure; density 2.477 g/cm3; stable in solid crystalline form; decomposes on heating, evolving oxygen; completely decomposes at about 100°C; sparingly soluble in cold water 1.75 g/100mL at 0°C; moderately soluble at ordinary temperature, 5.29 g/100 mL at 20°C;aqueous solution acidic and unstable, decomposing slowly at room temperature and more rapidly when the solution is warmed; insoluble in alcohol.
Uses
Potassium persulfate?is a powerful oxidant, commonly used to initiate polymerizations. Potassium Persulphate used for bleaching and textile desizing, as an oxidizing agent and antiseptic, in purification of ammonium sulfate, and in the manufacture of soap and pharmaceuticals. It is also used as al laboratory oxidant and photography chemical. It is a food additive.
Preparation
Potassium persulfate can be prepared by electrolysis of a mixture of potassium sulfate and potassium hydrogen sulfate at a high current density:
2KHSO4→K2S2O8+ H2
Also, the compound can be prepared by adding potassium hydrogen sulfate,KHSOto an electrolyzed solution of ammonium hydrogen sulfate, NH4HSO4.
General Description
A white crystalline solid. Specific gravity 2.477. Decomposes below 100°C.
Air & Water Reactions
Water soluble. Slowly decomposed by water. The salt rapidly liberates oxygen when heated, and especially so when wet.
Reactivity Profile
Potassium persulfate is an oxidizing agent. Noncombustible but accelerates the burning of combustible material. Potassium persulfate plus a little potassium hydroxide and water released sufficient heat and oxygen to ignite a polythene (polyethylene) liner in a container. [MCA Case History 1155. 1955].
Hazard
Strong irritant and oxidizing agent. Fire risk in contact with organic materials.
Health Hazard
Inhalation, ingestion or contact (skin, eyes) with vapors or substance may cause severe injury, burns or death. Fire may produce irritating, corrosive and/or toxic gases. Runoff from fire control or dilution water may cause pollution.
Fire Hazard
These substances will accelerate burning when involved in a fire. Some may decompose explosively when heated or involved in a fire. May explode from heat or contamination. Some will react explosively with hydrocarbons (fuels). May ignite combustibles (wood, paper, oil, clothing, etc.). Containers may explode when heated. Runoff may create fire or explosion hazard.
Agricultural Uses
Potassium sulphate, also called sulphate of potash, is a
white crystalline material, moderately hygroscopic,
available in fine, granular and semi-granular forms. It
contains 48 to 54% potassium (as K2O) and supplies 17 to
20 % of sulphate. Chloride-sensitive crops like tobacco,
grapes and potato require chloride-free potassium
fertilizers. Therefore, these crops are fertilized with
potassium sulphate, although this is more expensive than
potassium chloride. These three crops, being major
crops, account for about 7% of the total potash
consumption. For best results, potassium sulphate should
contain at least 50 % potash by weight.
Potassium sulphate occurs in nature as 'langbeinite' ,
a double sulphate of potassium and magnesium
(K2SO4?2MgSO4) and made from burkeite
(Na2CO3?2Na2SO4), kainite (KCl?MgS04?3H2O) or
potassium chloride (KCl) as follows:
When applied to soil, potassium ion from the watersoluble
potassium sulphate is retained in the soil colloids
and not easily leached out. This makes potassium
sulphate an excellent fertilizer, useful for all soils and
crops while sowing or before sowing. It is also a safe
ingredient of powdered, mixed fertilizers.
Safety Profile
Moderately toxic by ingestion. An irritant and allergen. A powerful oxidtzer. Flammable when exposed to heat or by chemical reaction. Can react with reducing materials. It liberates oxygen above 100' when dry or @ about 50' when in solution. When heated to decomposition it emits highly toxic fumes of SOx,, S2O8, and K2O.
Potential Exposure
Potassium persulfate is used as a bleaching and oxidizing agent; it is used in redox polymeri- zation catalysts; in the defiberizing of wet strength paper and in the desizing of textiles. Soluble in water.
Shipping
UN1492 Potassium persulfate, Hazard Class: 5.1; Labels: 5.1-Oxidizer.
Purification Methods
Crystallise the persulfate twice from distilled water (10mL/g) and dry it at 50o in a vacuum desiccator. Its solubility in H2O is 1.6% at 0o, 4.5% at 20o, and 7.2% at 30o. An aqueous solution decomposes on long standing with evolution of O2 and formation of KHSO4. It is a powerful oxidising agent. Store it at ~10o. [Fehér in Handbook of Preparative Inorganic Chemistry (Ed. Brauer) Academic Press Vol I p 390 1963.]
Incompatibilities
A strong oxidizer. Incompatible with combustible, organic or other readily oxidizable materials; sulfur, metallic dusts, such as aluminum dust; chlorates and perchlorates. Attacks chemically active metals. Keep away from moisture.
Waste Disposal
Use large volumes of reducing agents (bisulfites, e.g.). Neutralize with soda ash and drain into sewer with abundant water.
Potassium persulfate Preparation Products And Raw materials
Raw materials
Preparation Products
1of5
chevron_right| Supplier | Tel | Country | ProdList | Advantage | Inquiry |
|---|---|---|---|---|---|
| JSK Chemicals | +919879767970 | Gujarat, India | 3756 | 58 | Inquiry |
| Kronox Lab Sciences Pvt Ltd | +91-9313231074 +91-9313231074 | Gujarat, India | 240 | 58 | Inquiry |
| Ralington Pharma | +91-7948911722 +91-9687771722 | Gujarat, India | 1350 | 58 | Inquiry |
| Shri Shanti Laboratories | +91-9799144442 +91-9571511119 | Rajasthan, India | 45 | 58 | Inquiry |
| FINAR LTD | +91 - 2717 - 616717 | New Delhi, India | 660 | 58 | Inquiry |
| Maas Pharma Chemicals | +91-9958666224 | Delhi, India | 1607 | 58 | Inquiry |
| CLEARSYNTH LABS LTD. | +91-22-45045900 | Hyderabad, India | 6351 | 58 | Inquiry |
| Otto Chemie Pvt. Ltd. | +91 9820041841 | Mumbai, India | 5873 | 58 | Inquiry |
| Sisco Research Laboratories Pvt. Ltd. | +91-22-4268 5800 | Mumbai, India | 4317 | 58 | Inquiry |
| Alfa Aesar | 1 800 209 7001 | Maharashtra, India | 6913 | 58 | Inquiry |
| Supplier | Advantage |
|---|---|
| JSK Chemicals | 58 |
| Kronox Lab Sciences Pvt Ltd | 58 |
| Ralington Pharma | 58 |
| Shri Shanti Laboratories | 58 |
| FINAR LTD | 58 |
| Maas Pharma Chemicals | 58 |
| CLEARSYNTH LABS LTD. | 58 |
| Otto Chemie Pvt. Ltd. | 58 |
| Sisco Research Laboratories Pvt. Ltd. | 58 |
| Alfa Aesar | 58 |
7727-21-1(Potassium persulfate)Related Search:
1of4
chevron_right




