Benzoyl peroxide
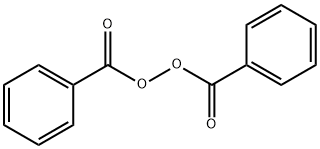
- CAS No.
- 94-36-0
- Chemical Name:
- Benzoyl peroxide
- Synonyms
- BPO;DIBENZOYL PEROXIDE;Benzoyl;benzoic peroxyanhydride;Benzoylperoxid;BENZOYL BENZENECARBOPEROXOATE;Benzamycin;Benzoyl peroide;Benzoylperoxyde;benzoicacidperoxide
- CBNumber:
- CB0484149
- Molecular Formula:
- C14H10O4
- Molecular Weight:
- 242.23
- MOL File:
- 94-36-0.mol
- Modify Date:
- 2024/7/7 20:48:41
| Melting point | 105 °C(lit.) |
|---|---|
| Boiling point | 176°F |
| Density | 1.16 g/mL at 25 °C(lit.) |
| vapor pressure | 0.009Pa at 25℃ |
| refractive index | 1.5430 (estimate) |
| Flash point | >230 °F |
| storage temp. | 2-8°C |
| solubility | 0.35mg/l |
| form | powder |
| color | White |
| Odor | odorless |
| Water Solubility | Insoluble |
| Merck | 14,1116 |
| BRN | 984320 |
| Exposure limits | TLV-TWA 5 mg/m3; IDLH 7000 mg/m3. |
| Stability | Strong oxidizer. Highly flammable. Do not grind or subject to shock or friction. Incompatible with reducing agents, acids, bases, alcohols, metals, organic materials. Contact with combustible material, heating or friction may cause fire or explosion. |
| InChIKey | OMPJBNCRMGITSC-UHFFFAOYSA-N |
| LogP | 3.2 at 20℃ |
| CAS DataBase Reference | 94-36-0(CAS DataBase Reference) |
| IARC | 3 (Vol. 36, Sup 7, 71) 1999 |
| NIST Chemistry Reference | Benzoyl peroxide(94-36-0) |
| EPA Substance Registry System | Benzoyl peroxide (94-36-0) |
SAFETY
Risk and Safety Statements
| Symbol(GHS) |     GHS02,GHS07,GHS08,GHS09 |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Signal word | Danger | |||||||||
| Hazard statements | H242-H317-H319-H360D-H410 | |||||||||
| Precautionary statements | P210-P235-P273-P280-P308+P313-P370+P378 | |||||||||
| Hazard Codes | O,Xn,N,Xi,E,T | |||||||||
| Risk Statements | 8-36/37/38-43-36-2-7-1-51/53-21/22-62-50-61-3-39/23/24/25-40 | |||||||||
| Safety Statements | 53-17-26-36/37-45-60-36/37/39-3/7-14A-14-47-35-7-61-37/39-24 | |||||||||
| RIDADR | UN 3108 5.2 | |||||||||
| OEB | B | |||||||||
| OEL | TWA: 5 mg/m3 | |||||||||
| WGK Germany | 2 | |||||||||
| RTECS | DM8575000 | |||||||||
| Autoignition Temperature | 176 °F | |||||||||
| TSCA | Yes | |||||||||
| HazardClass | 5.2 | |||||||||
| PackingGroup | II | |||||||||
| HS Code | 29163200 | |||||||||
| Toxicity | LD50 orally in Rabbit: 7710 mg/kg | |||||||||
| IDLA | 1,500 mg/m3 | |||||||||
| NFPA 704 |
|
Benzoyl peroxide price More Price(12)
| Manufacturer | Product number | Product description | CAS number | Packaging | Price | Updated | Buy |
|---|---|---|---|---|---|---|---|
| Sigma-Aldrich(India) | 8.01641 | Benzoyl peroxide (with 25% H?O) for synthesis | 94-36-0 | 10G | ₹1860 | 2022-06-14 | Buy |
| Sigma-Aldrich(India) | B5907 | Benzoyl peroxide blend with dicyclohexyl phthalate suitable for use as a catalyst for electron microscopy. Modified to render it safe in transit. | 94-36-0 | 1VIAL | ₹2381.5 | 2022-06-14 | Buy |
| Sigma-Aldrich(India) | 8.01641 | Benzoyl peroxide (with 25% H?O) for synthesis | 94-36-0 | 1KG | ₹9760 | 2022-06-14 | Buy |
| Sigma-Aldrich(India) | 8.01641 | Benzoyl peroxide (with 25% H?O) for synthesis | 94-36-0 | 26.7KG | ₹57539.99 | 2022-06-14 | Buy |
| Sigma-Aldrich(India) | 517909 | Luperox® A75, Benzoyl peroxide 75%, remainder water | 94-36-0 | 100G | ₹4037.73 | 2022-06-14 | Buy |
Benzoyl peroxide Chemical Properties,Uses,Production
Description
Benzoyl Peroxide may affect workers in the electronics and plastics (epoxy resins and catalysts) industries, electricians, ceramic workers, dentists and dental technicians, laboratory technicians and bakers. As it was contained in candles, it also induced contact dermatitis in a sacristan. However, some positive tests are of unknown occupational relevance.
Chemical Properties
Benzoyl peroxide is an odorless, white, or colorless crystalline powder with a faint odor of benzaldehyde resulting from the interaction of benzoyl chloride and a cooled sodium peroxide solution. It is used in specified cheeses at 0.0002% of milk level. It is also used for the bleaching of flour, slowly decomposing to exert its full bleaching action, which results in whiter flour and bread. The major decomposition product of benzoyl peroxide in water was benzoic acid, but trace amounts of phenyl benzoate, phenol, and hydroxybenzoic acids were formed. When carbon-14 la- beled benzoyl peroxide was reacted with whey, the same compounds were produced.
Uses
Benzoyl Peroxide is a widely used organic compound of the peroxide family. It is used as a source offree radicals in many organic syntheses andto initiate polymerizations of styrene, vinylchloride, vinyl acetate, and acrylics; to curethermoset polyester resins and silicone rubbers;in medicine for treating acne; and forbleaching vegetable oil, cheese, flour, and fats.
Indications
Benzoyl peroxide is a potent oxidizing agent that has both antimicrobial and comedolytic properties; its primary use is in treating acne vulgaris. It is converted in the skin to benzoic acid; clearance of absorbed drug is rapid, and no systemic toxicity has been observed. The major toxicities are irritation and contact allergy. Outgrowth of bacteria resistant to topical antibiotics used to treat acne can be reduced by the addition of benzoyl peroxide in combination products such as erythromycin (Benzamycin) and clindamycin (Benzaclin).
World Health Organization (WHO)
Benzoyl peroxide slowly releases oxygen and hence is bactericidal. It is also keratolytic, antiseborrheic and irritant. It is used in the treatment of acne. Benzoyl peroxide is listed in the WHO Model List of Essential Drugs.
General Description
Benzoyl peroxide appears as odorless white powder or granules. moderately toxic. Sinks in water. (USCG, 1999)
Reactivity Profile
Benzoyl peroxide reacts exothermically withstrong acids, strong bases, amines, reducingagents, and sulfur compounds. Explosionshave been reported when it reacted withcarbon tetrachloride and ethylene (Bolt andJoyce 1947), lithium aluminum hydride(Sutton 1951), N,N-dimethyl aniline (Hornerand Betzel 1953), hot chloroform (NFPA1986), and methyl methacrylate (NFPA1986). Lappin (1948) reported an explosionwhen a bottle was opened. Organic matterentrapped in the threads of the bottleprobably reacted explosively with benzoylperoxide.
Hazard
Highly toxic via inhalation. May explode spontaneously when dry (<1% of water). Never mix unless at least 33% water is present. Skin and upper respiratory tract irritant. Questionable carcinogen.
Health Hazard
The health hazard from benzoyl peroxideis low. It can cause irritation of the skin,mucous membranes, and eyes. An intraperitonealinjection of 250 mg/kg was lethal toadult mice. Systemic toxicity in humans isnot known. It may be mild to moderatelytoxic on an acute basis. The oral LD50 valuein rats is 7710 mg/kg (NIOSH 1986). Itstoxicity from inhalation is low; an LC50 valueof 700 ppm in mice is suggested (ACGIH1986).
Benzoyl peroxide may cause gene damageand DNA inhibition. It has been foundto cause skin tumor. The evidence of its carcinogenicityin animals and humans is inadequate.
Fire Hazard
Benzoyl peroxide can cause a major fire
and explosion hazard. It is highly flammable
and a strong oxidizer; autoignition temperature
80°C (176°F). It ignites instantly. The
rate and violence of decomposition and the
potential ease of such ignition or decomposition
have been experimentally measured
by Noller et al. (1964). Lead pipe deformation
(LPD), pressure vessel test (PVT), and
self-accelerating decomposition test (SADT)
have been performed to measure these explosive
characteristics. Heating 5 g of benzoyl
peroxide in an aluminum tester containing an
aperture vent and 6-atm rupture disk, caused
the disk to blow up in 95 seconds when the
aperture vent area was less than 174.7 mm2.
Redried material was more violent. The
decomposition hazard was greatly reduced
with wet and diluted benzoyl peroxide.
Noller et al. (1964) measured the SADT
temperature at 82.2°C (180°F), above which
the decomposition was self-accelerating, sudden,
and produced smoke.
Benzoyl peroxide is a deflagrant, posing
a severe explosion hazard. The compound
is sensitive to heavy shock, such as impact
or blows, as well as to friction and heat.
Especially in the dry state, it is highly
dangerous.
A water sprinkler should be used to extinguish
fires. Water should be used to keep the
containers cool.
Contact allergens
Benzoyl peroxide is an oxidizing agent widely employed in acne topical therapy. It is also used as a polymerization catalyst of dental or industrial plastics and as a decolorizing agent of flours, oils, fats, and waxes. Irritant or allergic dermatitis may affect workers in the electronics and plastics (epoxy resins and catalysts) industries, electricians, ceramic workers, dentists and dental technicians, laboratory technicians, bakers, and acne patients. As it was contained in candles, it also induced contact dermatitis in a sacristan. Patch tests may be irritant.
Safety Profile
Poison by intraperitoneal route.Can cause dermatitis, asthmatic effects, testicular atrophy,and vasodilation. An allergen and eye irritant. Humanmutation data reported. Questionable carcinogen withexperimental tumorigenic data. Moderate fire hazard by
Potential Exposure
Used as polymerization initiator, curing agent, and cross-linking agent.
Carcinogenicity
When repeatedly applied to the skin of mice, BPO was not carcinogenic . However, benzoyl peroxide is a tumor promoter in mice and hamsters, but has shown no complete carcinogenic or tumor-initiating activity . There has been one controversial Japanese report that was interpreted as BPO being a complete carcinogen. However, when the data were critically evaluated, it was found consistent with BPO acting as a skin tumor promoter and not as a carcinogen. The International Agency for Research on Cancer (IARC) has evaluated the carcinogenicity of benzoyl peroxide. They classified it as Group 3. This means there is limited or inadequate evidence of carcinogenicity for animals and inadequate or absent information for humans. In addition, there are other animals and in vitro studies that continue to support the lack of carcinogenic or mutagenic properties for BPO .
Source
Benzoyl peroxide (BPO) was originally derived from chlorhydroxyquinoline, a component of coal tar. Currently, BPO is usually prepared by treating hydrogen peroxide with benzoyl chloride.
storage
Benzoyl peroxide should be stored in acool and well-ventilated area, isolated fromother chemicals and free of heating andelectrical installations. Dry compound maybe shipped in polyethylene-lined paper bagsor fiber containers packed in wooden boxeso.
Shipping
UN3104 : Organic peroxide type C, solid, Hazard Class: 5.2; Labels: 5.2—Organic peroxide, Technical Name Required. UN3108 : Organic peroxide type E, solid, Hazard Class: 5.2; Labels: 5.2—Organic peroxide, Technical
Purification Methods
Dissolve benzoyl peroxide in CHCl3 at room temperature and precipitate it by adding an equal volume of MeOH or pet ether. Similarly it is precipitated from acetone by adding two volumes of distilled water. It has also been crystallised from 50% MeOH and from diethyl ether. Dry it under vacuum at room temperature for 24hours. Store it in a desiccator in the dark at 0o. When purifying in the absence of water it can be EXPLOSIVE, and operations should be done on a very small scale with adequate protection. Large amounts should be kept moist with water and stored in a refrigerator. [Kim et al. J Org Chem 52 3691 1987, Beilstein 9 IV 777.]
Incompatibilities
May explode when heated above melting point, 103 C. A strong oxidizer. Extremely explosionsensitive to heat, shock, friction, and concussion. May explode or cause fire on contact with reducing agents; combustible substances, organic substances, wood, paper, metal powders, lithium aluminum hydride. Violent reaction with alcohols, organic and inorganic acids, and amines.
Waste Disposal
Pretreatment involves decomposition with sodium hydroxide. The final solution of sodium benzoate, which is very biodegradable, may be flushed into the drain. Disposal of large quantities of solution may require pH adjustment before release into the sewer or controlled incineration after mixing with a noncombustible material.
Benzoyl peroxide Preparation Products And Raw materials
Raw materials
1of2
chevron_rightPreparation Products
1of8
chevron_right| Supplier | Tel | Country | ProdList | Advantage | Inquiry |
|---|---|---|---|---|---|
| CLEARSYNTH LABS LTD. | +91-22-45045900 | Mumbai, India | 66 | 58 | Inquiry |
| Nikava Pharmaceutical Industries | +91-9653317212 +91-9653317212 | Mumbai, India | 20 | 58 | Inquiry |
| Geeta Intermediates | +91-9422337733 +91-9422337733 | Maharashtra, India | 1 | 58 | Inquiry |
| Pd Navkar Bio Chem Pvt Ltd | +91-8022157444 +91-8022157444 | Karnataka, India | 49 | 58 | Inquiry |
| Zydus Lifesciences Ltd. | +91-0265-2315243 +91-6358895661 | Gujarat, India | 89 | 58 | Inquiry |
| Dhiraj Chemicals | +91-9822871166 +91-9819681166 | Maharashtra, India | 1 | 58 | Inquiry |
| DeFINE CHEMICALS | +91-8010769889 +91-9320057099 | Maharashtra, India | 120 | 58 | Inquiry |
| Plasti Pigments Pvt Ltd | +91 9664679993 | Mumbai, India | 7 | 58 | Inquiry |
| Veekay Chemicals | +91-8657432590 +91-9821518903 | Maharashtra, India | 37 | 58 | Inquiry |
| Abhinav Polychem Pvt Ltd | +91-9967188059 +91-8779799643 | Maharashtra, India | 3 | 58 | Inquiry |
| Supplier | Advantage |
|---|---|
| CLEARSYNTH LABS LTD. | 58 |
| Nikava Pharmaceutical Industries | 58 |
| Geeta Intermediates | 58 |
| Pd Navkar Bio Chem Pvt Ltd | 58 |
| Zydus Lifesciences Ltd. | 58 |
| Dhiraj Chemicals | 58 |
| DeFINE CHEMICALS | 58 |
| Plasti Pigments Pvt Ltd | 58 |
| Veekay Chemicals | 58 |
| Abhinav Polychem Pvt Ltd | 58 |
Related articles
- Efficacy and side effects of Benzoyl peroxide in the treatment of acne
- Benzoyl peroxide (BPO) is one of the most widely used topical acne medications. It has potent antibacterial, mild anti-inflamm....
- Jul 5,2024
- Benzoyl peroxide: a widely used organic acid
- Benzoyl peroxide is an organic acid with widely used
- Sep 22,2023
- Benzoyl peroxide: Medical use, mechanism and side effects
- Benzoyl Peroxide is a peroxide with antibacterial, irritant, keratolytic, comedolytic, and anti-inflammatory activity.
- May 16,2023
94-36-0(Benzoyl peroxide)Related Search:
1of4
chevron_right




