Di-tert-butyl peroxide
- CAS No.
- 110-05-4
- Chemical Name:
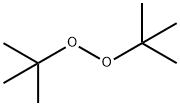
- Di-tert-butyl peroxide
- Synonyms
- DTBP;2-(tert-Butylperoxy)-2-methylpropane;TERT-BUTYL PEROXIDE;DI-T-BUTYL PEROXIDE;Trigonox b;(tributyl)peroxide;bis(tert-butyl)peroxide;DI-TERTIARY-BUTYL PEROXIDE;Cadox;cadoxtbp
- CBNumber:
- CB8852799
- Molecular Formula:
- C8H18O2
- Molecular Weight:
- 146.23
- MOL File:
- 110-05-4.mol
- MSDS File:
- SDS
- Modify Date:
- 2023/12/22 17:53:22
| Melting point | -30 °C |
|---|---|
| Boiling point | 109-110 °C(lit.) |
| Density | 0.796 g/mL at 25 °C(lit.) |
| vapor pressure | 40 mm Hg ( 20 °C) |
| refractive index |
n |
| Flash point | 34 °F |
| storage temp. | Store at +15°C to +25°C. |
| solubility | 0.063g/l |
| form | Liquid |
| color | Clear |
| Odor | distinctive odor |
| Water Solubility | immiscible |
| Merck | 14,3461 |
| BRN | 1735581 |
| Stability | May decompose explosively if heated, subjected to shock, or treated with reducing agents. Highly flammable. Refrigerate. |
| InChIKey | LSXWFXONGKSEMY-UHFFFAOYSA-N |
| LogP | 3.2 at 22℃ |
| CAS DataBase Reference | 110-05-4(CAS DataBase Reference) |
| NIST Chemistry Reference | Di-tert-butyl peroxide(110-05-4) |
| EPA Substance Registry System | Di-tert-butyl peroxide (110-05-4) |
SAFETY
Risk and Safety Statements
| Symbol(GHS) |   GHS02,GHS08 |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Signal word | Danger | |||||||||
| Hazard statements | H225-H242-H341-H412 | |||||||||
| Precautionary statements | P210-P220-P273-P280-P410-P411+P235 | |||||||||
| Hazard Codes | O,F,Xn | |||||||||
| Risk Statements | 7-11-68-52/53-2017/7/11 | |||||||||
| Safety Statements | 14-16-3/7-36/37/39-14A-61-23 | |||||||||
| RIDADR | UN 3107 5.2 | |||||||||
| WGK Germany | 1 | |||||||||
| RTECS | ER2450000 | |||||||||
| Autoignition Temperature | 182 °C | |||||||||
| HS Code | 2909 60 00 | |||||||||
| HazardClass | 5.2 | |||||||||
| PackingGroup | II | |||||||||
| Toxicity | LD50 orally in Rabbit: > 25000 mg/kg | |||||||||
| NFPA 704 |
|
Di-tert-butyl peroxide price More Price(8)
| Manufacturer | Product number | Product description | CAS number | Packaging | Price | Updated | Buy |
|---|---|---|---|---|---|---|---|
| Sigma-Aldrich | 168521 | Luperox® DI, tert-Butyl peroxide 98% | 110-05-4 | 5ML | ₹3767.1 | 2022-06-14 | Buy |
| Sigma-Aldrich(India) | 168521 | Luperox® DI, tert-Butyl peroxide 98% | 110-05-4 | 5ML | ₹3767.1 | 2022-06-14 | Buy |
| Sigma-Aldrich | 168521 | Luperox® DI, tert-Butyl peroxide 98% | 110-05-4 | 250ML | ₹5726.43 | 2022-06-14 | Buy |
| Sigma-Aldrich(India) | 168521 | Luperox® DI, tert-Butyl peroxide 98% | 110-05-4 | 250ML | ₹5726.43 | 2022-06-14 | Buy |
| Sigma-Aldrich(India) | 8.20248 | Di-tert-butyl peroxide for synthesis | 110-05-4 | 1L | ₹36590 | 2022-06-14 | Buy |
Di-tert-butyl peroxide Chemical Properties,Uses,Production
Description
Di-tert-butyl peroxide is a clear, water-white liquid. It has a specific gravity of 0.79, which is lighter than water, and it will float on the surface. It is nonpolar and insoluble in water. Di-tert-butyl peroxide is a strong oxidizer and may ignite organic materials or explode if shocked or in contact with reducing agents. In addition to being an oxidizer, Di-tert-butyl peroxide is highly flammable. It has a boiling point of 231°F (110°C) and a flash point of 65°F (18°C). The NFPA 704 designation is health 3, flammability 2, and reactivity 4. The prefix “oxy” for oxidizer is placed in the white section at the bottom of the 704 diamond.
Chemical Properties
Di-tert-butyl peroxide (DTBP) consists of a peroxide group bonded to two tert-butyl groups. Since the tert-butyl groups are bulky, it is one of the most stable organic peroxides.
Uses
The decomposition reaction proceeds via the generation of methyl radicals. The peroxide bond undergoes homolysis at temperatures above 100 °C. Hence di-tert-butyl peroxide is commonly used as a radical initiator in organic synthesis and polymer chemistry. DTBP can in principle be used in engines where oxygen is limited, since the molecule supplies both the oxidizer and the fuel.
Uses
Di-tert-butyl peroxide has been used as a radical initiator to induce free radical polymerization. It has also been used as a cetane enhancer in a study to determine the phase behavior of carboxylate-based extended surfactant reverse micellar microemulsions with ethanol and vegetable oil/diesel blends.
General Description
Di-tert-butyl peroxide is a clear colorless liquid. (NTP, 1992)
Reactivity Profile
The explosive instability of the lower dialkyl peroxides (e.g., dimethyl peroxide) and 1,1-bis-peroxides decreases rapidly with increasing chain length and degree of branching, the di-tert-alkyl derivatives being amongst the most stable class of peroxides. Though many 1,1-bis-peroxides have been reported, few have been purified because of the higher explosion hazards compared with the monofunctional peroxides. Di-tert-butyl peroxide is unlikely that this derivative would be particularly unstable compared to other peroxides in it's class, Bretherick 1979v.
Health Hazard
DTBP is slightly toxic by inhalation andin general exhibits low to very low toxicityby other routes. However, toxic effectsare observed only at very high concentrations.Rats exposed to 4103-ppm vapor developedhead and neck tremor after 10 minutesof exposure (Floyd and Stockinger 1958).Other symptoms were weakness, hyperactivity,and labored breathing. However, theanimals recovered fully in 1 hour.
LD50 value, intraperitoneal rats): 3210 mg/kg
DTBP is nonirritating to the skin and mildon the eyes. It is reported to cause lungand blood tumors in mice (NIOSH 1986).However, its carcinogenicity is not yet fullyestablished.
Fire Hazard
Highly flammable and reactive; flash point
18°C (64.4°F); vapor pressure 19.5 torr at
20°C (68°F); vapor density 5.03. Its decomposition
products are ethane and acetone,
which enhance the fire hazard. Use a water
spray to fight fire and to keep the containers
cool.
DTBP forms an explosive mixture with
air. The explosive range is not reported. Its
decomposition products may explode above
its boiling point, 111°C (231.8°F). However,
as it is thermally stable and shock insensitive,
its explosion hazard is much lower. It may,
however, react with explosive violence when
in contact with easily oxidizable substances.
Carcinogenicity
A single exposure (route unspecified, but probably subcutaneous (SC)) of 14.6 mg (~365 mg/kg) produced unconvincing evidence for carcinogenicity owing to the lack of controls in 50 mice observed for more than 80 weeks. Of 35 survivors, 7 (20%) had malignant blood tumors (lymphomas) and 1 had a benign lung tumor (pulmonary adenoma) (93, 7a). Owing to its poor design, this study should be judged inadequate to determine carcinogenicity.
storage
Store in a cool and well-ventilated areaisolated from easily oxidizable materials.Protect against physical damage. Shippingcontainers are amber glass and polyethylenebottles or steel drums not exceeding 100-lbcapacity.
Purification Methods
Wash the peroxide with aqueous AgNO3 to remove olefinic impurities, water and dry (MgSO4). Free it from tert-butyl hydroperoxide by passage through an alumina column [Jackson et al. J Am Chem Soc 107 208 1985], and if necessary two high vacuum distillations from room temperature to a liquid-air trap [Offenbach & Tobolsky J Am Chem Soc 79 278 1957]. [Beilstein 1 IV 1619.] The necessary protection from EXPLOSION should be used.
Waste Disposal
Di-tert-butyl peroxide is disposed on the ground in a remotearea and ignited with a long torch. 10%NaOH may be used to wash empty containers.
Di-tert-butyl peroxide Preparation Products And Raw materials
Raw materials
Preparation Products
1of4
chevron_right| Supplier | Tel | Country | ProdList | Advantage | Inquiry |
|---|---|---|---|---|---|
| Plasti Pigments Pvt Ltd | +91 9664679993 | Mumbai, India | 7 | 58 | Inquiry |
| Veekay Chemicals | +91-8657432590 +91-9821518903 | Maharashtra, India | 37 | 58 | Inquiry |
| Samuh Laxmi Chemicals (Bom) P Ltd | +91-9004585338 +91-8369298209 | Maharashtra, India | 25 | 58 | Inquiry |
| Ambani Organics Limited | +91-2226822027 +91-2226822027 | Maharashtra, India | 8 | 58 | Inquiry |
| CLEARSYNTH LABS LTD. | +91-22-45045900 | Hyderabad, India | 6351 | 58 | Inquiry |
| Dhingra Plastic And Plasticisers (P) Ltd | 08048978503 | Haryana, India | 6 | 58 | Inquiry |
| United Engineers | 08048372765Ext 944 | Mumbai, India | 3 | 58 | Inquiry |
| Dhingra Plastic & Plastisciers Pvt Ltd | 08048361276Ext 238 | New Delhi, India | 1 | 58 | Inquiry |
| Om Organic Chemicals | 08048372505Ext 687 | Maharashtra, India | 5 | 58 | Inquiry |
| Scieno-Chem LLP. | 08048985497 | Maharashtra, India | 1 | 58 | Inquiry |
Related articles
- Di-tert-butyl peroxide: properties and applications in organic synthesis
- Di-tert-butyl peroxide is a stable peroxide used as a radical initiator in polymerization and alkylating reactions, facilitati....
- Aug 2,2023
- What is Di-tert-butyl peroxide?
- Di-tert-butyl peroxide is used for synthesis. CAS 110-05-4, molar mass 146.23 g/mol.
- Jul 9,2021





