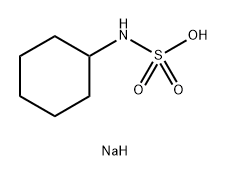
Sodium N-cyclohexylsulfamate
- CAS No.
- 139-05-9
- Chemical Name:
- Sodium N-cyclohexylsulfamate
- Synonyms
- SODIUM CYCLAMATE;CYCLAMATE;CYCLAMATE SODIUM;N-CYCLOHEXYLSULFAMIC ACID SODIUM SALT;asugryn;ibiosuc;sucrum7;sugarin;sugaron;cyclamic
- CBNumber:
- CB8713674
- Molecular Formula:
- C6H14NNaO3S
- Molecular Weight:
- 203.23
- MOL File:
- 139-05-9.mol
- MSDS File:
- SDS
- Modify Date:
- 2024/8/21 22:41:43
| Melting point | >300 °C (lit.) |
|---|---|
| Density | 1.58[at 20℃] |
| vapor pressure | 0.002Pa at 150℃ |
| storage temp. | room temp |
| solubility | 200g/l |
| form | Powder |
| color | White |
| Odor | Odorless. Sweet taste, perceptible at concentrations higher than 100 ppm in water. |
| PH | 5.5-7.5 (100g/l, H2O, 20℃) |
| Water Solubility | >=10 g/100 mL at 20 ºC |
| Merck | 14,2703 |
| BRN | 4166868 |
| Stability | Hygroscopic |
| InChIKey | UDIPTWFVPPPURJ-UHFFFAOYSA-M |
| LogP | -2.63 at 20℃ |
| CAS DataBase Reference | 139-05-9(CAS DataBase Reference) |
| IARC | 3 (Vol. Sup 7, 73) 1999 |
| EPA Substance Registry System | Sodium cyclamate (139-05-9) |
SAFETY
Risk and Safety Statements
| Symbol(GHS) |  GHS07 |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Signal word | Warning | |||||||||
| Hazard statements | H302 | |||||||||
| Precautionary statements | P264-P270-P501-P301+P312a-P330-P501a-P301+P312+P330 | |||||||||
| Hazard Codes | Xn | |||||||||
| Risk Statements | 22 | |||||||||
| Safety Statements | 36/37-24/25 | |||||||||
| WGK Germany | 3 | |||||||||
| RTECS | GV7350000 | |||||||||
| TSCA | Yes | |||||||||
| HS Code | 29299000 | |||||||||
| Toxicity | Sodium cyclamate is a non-nutritive sweetener that is about 30 times sweeter than cane sugar. The oral LD50s in rats and mice are 15.25 and 17.0 g/kg, respectively. It was banned as a food additive because of findings that it caused bladder cancer in rodents. It appears to act as a promoter. There is also evidence that it causes toxicity in the male reproductive system. | |||||||||
| NFPA 704 |
|
Sodium N-cyclohexylsulfamate price More Price(8)
| Manufacturer | Product number | Product description | CAS number | Packaging | Price | Updated | Buy |
|---|---|---|---|---|---|---|---|
| Sigma-Aldrich(India) | 71440 | Sodium N-cyclohexylsulfamate ≥99.0% (T) | 139-05-9 | 250G | ₹4643.93 | 2022-06-14 | Buy |
| Sigma-Aldrich | 47827 | Sodium Cyclamate analytical standard | 139-05-9 | 1000MG | ₹3615.55 | 2022-06-14 | Buy |
| Sigma-Aldrich(India) | 47827 | Sodium Cyclamate analytical standard | 139-05-9 | 1000MG | ₹3615.55 | 2022-06-14 | Buy |
| ALFA India | ALF-A18666-30 | Sodium cyclamate, 98% | 139-05-9 | 250g | ₹4500 | 2022-05-26 | Buy |
| ALFA India | ALF-A18666-0I | Sodium cyclamate, 98% | 139-05-9 | 5000g | ₹24508 | 2022-05-26 | Buy |
Sodium N-cyclohexylsulfamate Chemical Properties,Uses,Production
Chemical Properties
White powder
History
Cyclamate was first synthesized in 1937. Like the other sweeteners, its sweet taste was accidentally discovered (U.S. Pat. 2,275,125 (Mar. 3, 1942), L. F. Andrieth and M. Sveda (to E. I. du Pont de Nemours & Co., Inc.). The FDA in 1958 classified sodium cyclamate as a GRAS sweetener. In 1969, a 2-year chronic toxicity study with a sodium cyclamate–sodium saccharin (10:1) mixture found bladder tumors in rats. The FDA took cyclamate off the GRAS list, banning it from foods and beverages, but permitting its sale in pharmacies. In 1970, after a congressional investigation, the FDA banned the use of cyclamate entirely. Abbott Laboratories, which has conducted additional toxicity and carcinogenicity studies with cyclamate, a 10:1 mixture of cyclamate–saccharin, and cyclohexylamine, claimed to be unable to confirm the 1969 findings. Abbott then filed a food additive petition for cyclamate in 1973, which was denied by the FDA in 1980. In 1982, the Calorie Control Council and Abbott Laboratories filed a second food additive petition containing the results of additional safety studies (The Calorie Control Council and Abbott Laboratories, Food Additive Petition for cyclamate 2A3672 (Sept. 22, 1982). That petition remains active.
Production Methods
Cyclamates are prepared by the sulfonation of cyclohexylamine in the presence of a base. Commercially, the sulfonation can involve sulfamic acid, a sulfate salt, or sulfur trioxide. Tertiary bases such as triethylamine or trimethylamine may be used as the condensing agent. The amine salts of cyclamate that are produced are converted to the sodium, calcium, potassium, or magnesium salt by treatment with the appropriate metal oxide.
World Health Organization (WHO)
Cyclamates, non-nutritive sweetening agents, have been used as additives in food and drugs since 1950. They have been demonstrated to have a carcinogenic potential at very high and long-sustained dosage in experimental animals. Some countries have consequently banned their use as food additives, whereas in others they remain available for this purpose. Most countries, however, continue to allow their use in small quantities in pharmaceutical preparations. (Reference: (WHODI) WHO Drug Information, 77.2, 12, 1977)
General Description
Odorless or almost odorless white crystals or crystalline powder. Intensely sweet taste, even in dilute solution. pH (10% solution in water): 5.5-7.5. Used as a non-nutritive sweetener.
Air & Water Reactions
Water soluble.
Reactivity Profile
Sodium N-cyclohexylsulfamate is incompatible with strong oxidizing agents, strong acids and strong bases. Also incompatible with nitrites in acid solution. Has only limited compatibility with potassium salts .
Hazard
Some evidence of causing cancer in lab- oratory animals. Prohibited by FDA for food use. Questionable carcinogen.
Fire Hazard
Flash point data for Sodium N-cyclohexylsulfamate are not available; however, Sodium N-cyclohexylsulfamate is probably combustible.
Pharmaceutical Applications
Sodium cyclamate is used as an intense sweetening agent in pharmaceutical formulations, foods, beverages, and table-top sweeteners. In dilute solution, up to about 0.17% w/v, the sweetening power is approximately 30 times that of sucrose. However, at higher concentrations this is reduced and at a concentration of 0.5% w/v a bitter taste becomes noticeable. Sodium cyclamate enhances flavor systems and can be used to mask some unpleasant taste characteristics. In most applications, sodium cyclamate is used in combination with saccharin, often in a ratio of 10 : 1.
Toxicology
Sodium cyclamate is an odorless powder. It is about 30 times as sweet as sucrose in dilute solution. The structure of sodium cyclamate is shown in Figure 10.10 Capillary transitional cell tumors were found in the urinary bladders of 8 out of 80 rats that received 2600 mg/kg body weight per day of a mixture of sodium cyclamate and sodium saccharin (10:1) for up to 105 weeks. When the test mixture was fed at dietary levels designed to furnish 500, 1120, and 2500 mg/ kg body weight to groups of 35 and 45 female rats, the only significant finding was the occurrence of papillar carcinomas in the bladders of 12 of 70 rats fed the maximum dietary level of the mixture (equivalent of about 25 g/kg body weight) for periods ranging from 78 to 105 weeks (except for one earlier death). In vivo conversion from sodium cyclamate to cyclohexylamine was observed particularly in the higher dosage group. Cyclohexylamine is very toxic (LD50 rat oral=157 mg/dg) compared to sodium cyclamate (LD50 oral=12g/kg).
Safety Profile
Moderately toxic by intravenous and intraperitoneal routes. Mildly toxic by ingestion. Experimental reproductive effects. Questionable carcinogen with experimental neoplastigenic, tumorigenic, and teratogenic data. Human mutation data reported. When heated to decomposition it emits very toxic fumes of NazO, SOx, and NOx.
storage
Sodium cyclamate is hydrolyzed by sulfuric acid and cyclohexylamine
at a very slow rate that is proportional to the hydrogen ion
concentration. Therefore, for all practical considerations, it can be
regarded as stable. Solutions are also stable to heat, light, and air
over a wide pH range.
Samples of tablets containing sodium cyclamate and saccharin
have shown no loss in sweetening power following storage for up to
20 years.
The bulk material should be stored in a well-closed container in a
cool, dry place.
Application
Sodium N-cyclohexylsulfamate (SCHS) dissolved in the aqueous phase could be introduced as the additive during the interfacial polymerization process. SCHS could not only improve the hydrophilicity and flux of the membrane, but also could enhance the anti-fouling properties of the membrane[1].
References
[1] Chen G, et al. Fabrication and characterization of a novel nanofiltration membrane by the interfacial polymerization of 1,4-diaminocyclohexane (DCH) and trimesoyl chloride (TMC). RSC Advances, 2015; 5: 40742-40752.
Regulatory Status
The use of cyclamates as artificial sweetners in food, soft drinks, and
artificial sweetening tablets was at one time prohibited in the UK
and some other countries owing to concern about the metabolite cyclohexylamine. However, this is no longer the case, and
cyclamates are now permitted for use as a food additive in Europe.
Included in the FDA Inactive Ingredients Database (oral powder,
solutions, chewable tablets, and suspensions). Included in nonparenteral
medicines licensed in the UK. Included in the Canadian List
of Acceptable Non-medicinal Ingredients.
Sodium N-cyclohexylsulfamate Preparation Products And Raw materials
Raw materials
1of2
chevron_rightPreparation Products
| Supplier | Tel | Country | ProdList | Advantage | Inquiry |
|---|---|---|---|---|---|
| Vijaya Pharma And Life Science | +91-8939866271 +91-8939866271 | Tamil Nadu, India | 320 | 58 | Inquiry |
| Shree Paras Overseas | 07942540703 | Delhi, India | 1 | 58 | Inquiry |
| TCI Chemicals (India) Pvt. Ltd. | 1800 425 7889 | New Delhi, India | 6778 | 58 | Inquiry |
| Alfa Aesar | 1 800 209 7001 | Maharashtra, India | 6913 | 58 | Inquiry |
| Pharmaffiliates Analytics and Synthetics P. Ltd | +91-172-5066494 | Haryana, India | 6773 | 58 | Inquiry |
| Prakash Chemicals International Private Limited | 91-265-6126197 | New Delhi, India | 204 | 58 | Inquiry |
| Opulent Pharma | 08068441147Ext 981 | Gujarat, India | 810 | 58 | Inquiry |
| Pharma Affiliates | 172-5066494 | Haryana, India | 6761 | 58 | Inquiry |
| Pharmaffiliates Analytics & Synthetics (P) Ltd. | 91-172-5066494 | Haryana, India | 631 | 58 | Inquiry |
| Clearsynth Labs | 91-22-45045900 | Maharashtra, India | 3889 | 58 | Inquiry |
Related articles
- Sodium Cyclamate: A Deep Dive into Its Uses and Safety in Modern Chemistry
- Sodium cyclamate has gained significant attention in the chemical and food industries due to its potent sweetness and cost-eff....
- Aug 19,2024





