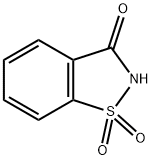
Saccharin
- CAS No.
- 81-07-2
- Chemical Name:
- Saccharin
- Synonyms
- SACCHARINE;Benzo[d]isothiazol-3(2H)-one 1,1-dioxide;O-SULFOBENZIMIDE;INSOLUBLE SACCHARIN;GLUCID;GLUSIDE;Sacharin;GARANTOSE;Benzosulfimide;SYNCAL (R) SDI
- CBNumber:
- CB1743735
- Molecular Formula:
- C7H5NO3S
- Molecular Weight:
- 183.18
- MOL File:
- 81-07-2.mol
- MSDS File:
- SDS
- Modify Date:
- 2024/5/28 19:59:05
| Melting point | 226-229 °C (lit.) |
|---|---|
| Boiling point | subl |
| Density | 0.828 |
| vapor pressure | 0Pa at 25℃ |
| refractive index | 1.5500 (estimate) |
| storage temp. | Store below +30°C. |
| solubility | acetone: soluble1g in 12mL(lit.) |
| pka | 11.68(at 18℃) |
| form | Crystals or Crystalline Powder |
| color | White |
| Odor | odorless |
| Water Solubility | 3.3 g/L (20 ºC) |
| Merck | 14,8311 |
| BRN | 6888 |
| Stability | Stable. Incompatible with strong oxidizing agents. |
| InChIKey | CVHZOJJKTDOEJC-UHFFFAOYSA-N |
| LogP | -0.024 at 25℃ |
| CAS DataBase Reference | 81-07-2(CAS DataBase Reference) |
| IARC | 3 (Vol. Sup 7, 73) 1999 |
| NIST Chemistry Reference | Saccharin(81-07-2) |
| EPA Substance Registry System | Saccharin (81-07-2) |
SAFETY
Risk and Safety Statements
| Symbol(GHS) |   GHS07,GHS08 |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Signal word | Warning | |||||||||
| Hazard statements | H302-H315-H319-H335-H351-H361 | |||||||||
| Precautionary statements | P261-P305+P351+P338 | |||||||||
| Risk Statements | 40-62-63-68 | |||||||||
| Safety Statements | 24/25 | |||||||||
| RIDADR | UN 3077 9/PG 3 | |||||||||
| WGK Germany | 2 | |||||||||
| RTECS | DE4200000 | |||||||||
| TSCA | Yes | |||||||||
| HazardClass | IRRITANT | |||||||||
| HS Code | 29251100 | |||||||||
| Toxicity | LD50 oral in mouse: 17gm/kg | |||||||||
| NFPA 704 |
|
Saccharin price More Price(16)
| Manufacturer | Product number | Product description | CAS number | Packaging | Price | Updated | Buy |
|---|---|---|---|---|---|---|---|
| Sigma-Aldrich(India) | 8.20128 | Saccharin for synthesis | 81-07-2 | 250G | ₹7220 | 2022-06-14 | Buy |
| Sigma-Aldrich(India) | PHR1341 | Saccharin Pharmaceutical Secondary Standard; Certified Reference Material | 81-07-2 | 1G | ₹8107.93 | 2022-06-14 | Buy |
| Sigma-Aldrich(India) | 8.20128 | Saccharin for synthesis | 81-07-2 | 1KG | ₹15210 | 2022-06-14 | Buy |
| Sigma-Aldrich(India) | 8.20128 | Saccharin for synthesis | 81-07-2 | 5KG | ₹56480 | 2022-06-14 | Buy |
| Sigma-Aldrich(India) | 240931 | Saccharin ≥99% | 81-07-2 | 1G | ₹3204.2 | 2022-06-14 | Buy |
Saccharin Chemical Properties,Uses,Production
Chemical Properties
Saccharin is a crystalline solid with a sweet taste (500 times sweeter than sugar).
Uses
It is a non-nutritive sweetener; pharmaceutic aid (flavor). Saccharin was formerly listed as reasonably anticipated to be a human carcinogen; delisted because the cancer data are not sufficient to meet the current criteria for this listing.
Definition
saccharin: A white crystalline solid,C7H5NO3S, m.p. 224°C. It is madefrom a compound of toluene, derivedfrom petroleum or coal tar. It is awell-known artificial sweetener,being some 500 times as sweet assugar (sucrose), and is usually marketedas its sodium salt. Because ofan association with cancer in laboratoryanimals, its use is restricted insome countries.
Preparation
Saccharin is synthesized using two methods: the Remsen-Fahlberg process and the Maumee or Sherwin-Williams method. The Remsen-Fahlberg synthesis of saccharin starts by reacting toluene with chlorosulfonic acid to give ortho and para forms of toluene-sulfonic acid (Figure 78.1). The acid can be converted to sulfonyl chlorides by treating with phosphorus pentachloride. The ortho form, o-toluene-sulfonyl chloride, is treated with ammonia to give o-toluene-sulfonamide, which is then oxidized with potassium permanganate to produce o-sulfamido-benzoic acid. On heating, the latter yields saccharin. Another synthesis was developed at Maumee Chemical Company in Toledo, Ohio, and it came to be known as the Maumee process. This process starts with phthalic anhydride, which is converted into anthranilic acid. Anthranilic acid is then reacted with nitrous acid, sulfur dioxide, chlorine, and ammonia to give saccharin. The Maumee process was further refi ned by the Sherwin-Williams Company and is therefore now referred to as the Sherwin-Williams process.
Production Methods
Saccharin is prepared from toluene by a series of reactions known as the Remsen–Fahlberg method. Toluene is first reacted with chlorosulfonic acid to form o-toluenesulfonyl chloride, which is reacted with ammonia to form the sulfonamide. The methyl group is then oxidized with dichromate, yielding o-sulfamoylbenzoic acid, which forms the cyclic imide saccharin when heated.
An alternative method involves a refined version of the Maumee process. Methyl anthranilate is initially diazotized to form 2- carbomethoxybenzenediazonium chloride; sulfonation followed by oxidation then yields 2-carbomethoxybenzenesulfonyl chloride. Amidation of this material, followed by acidification, forms insoluble acid saccharin.
General Description
White crystals. Odorless or faintly aromatic odor. Sweet taste.
Air & Water Reactions
Slightly soluble in water.
Reactivity Profile
An amide. Acid to litmus. pH of 0.35% aqueous solution: 2.0. Organic amides/imides react with azo and diazo compounds to generate toxic gases. Flammable gases are formed by the reaction of organic amides/imides with strong reducing agents. Amides are very weak bases (weaker than water). Imides are less basic yet and in fact react with strong bases to form salts. That is, they can react as acids. Mixing amides with dehydrating agents such as P2O5 or SOCl2 generates the corresponding nitrile. The combustion of these compounds generates mixed oxides of nitrogen (NOx).
Hazard
A questionable carcinogen. Products con- taining it must have a warning label.
Fire Hazard
Flash point data for Saccharin are not available; however, Saccharin is probably combustible.
Pharmaceutical Applications
Saccharin is an intense sweetening agent used in beverages, food
products, table-top sweeteners, and oral hygiene products such as
toothpastes and mouthwashes. In oral pharmaceutical formulations,
it is used at a concentration of 0.02–0.5% w/w. It has been
used in chewable tablet formulations as a sweetening agent.
Saccharin has been used to form various pharmaceutical cocrystals.
Saccharin can be used to mask some unpleasant taste characteristics
or to enhance flavor systems. Its sweetening power is
approximately 300–600 times that of sucrose.
Safety Profile
Confirmed carcinogen withexperimental neoplastigenic and tumorigenic data. Mildacute toxicity by ingestion. Experimental teratogenic andreproductive effects. Mutation data reported. Whenheated to decomposition it emits toxic NOx and SOx.
Potential Exposure
The information provided has to do, primarily, with the manufacturing of saccharin. Saccharin has been used as a nonnutritive sweetening agent. At one point the United States consumption pattern for all forms of saccharin has been estimated as 45% in soft drinks; 18% in tabletop sweeteners; 14% in fruits, juices, sweets, chew- ing gum, and jellies; 10% in cosmetics and oral hygiene products; 7% in drugs, such as coating on pills; 2% in tobacco; 2% in electroplating; and 2% for miscellaneous uses. Human exposure to saccharin occurs primarily through ingestion because of its use in many dietic foods and drinks and some personal hygiene products, including toothpastes and mouthwashes. The general public is exposed to saccharin, especially by persons required to reduce sugar intake.
storage
Saccharin is stable under the normal range of conditions employed
in formulations. In the bulk form it shows no detectable
decomposition and only when it is exposed to a high temperature
(125°C) at a low pH (pH 2) for over 1 hour does significant
decomposition occur. The decomposition product formed is
(ammonium-o-sulfo)benzoic acid, which is not sweet. The
aqueous stability of saccharin is excellent.
Saccharin should be stored in a well-closed container in a dry
place.
Shipping
UN3077 Environmentally hazardous substances, solid, n.o.s., Hazard class: 9; Labels: 9-Miscellaneous haz- ardous material, Technical Name Required.
Purification Methods
Purify saccharin by recrystallisation from Me2CO [solubility 7.14% at 0o, 14.4% at 50o], or aqueous isoPrOH to give a fluorescent solution. It sublimes in vacuo. It is an artificial sweetner and is 500 times sweeter than sucrose. [DeGarmo et al. J Am Pharm Assoc (Sci Ed) 41 17 1952, Beilstein 27 H 168, 870, 27 I 266, 27 II 214, 27 III/IV 2649.]
Incompatibilities
Saccharin can react with large molecules, resulting in a precipitate being formed. It does not undergo Maillard browning.
Waste Disposal
Consult with environmental regulatory agencies for guidance on acceptable disposal practices. Generators of waste containing this contami- nant (≥100 kg/mo) must conform to EPA regulations governing storage, transportation, treatment, and waste disposal.
Regulatory Status
Accepted for use as a food additive in Europe. Note that the EU number ‘E954’ is applied to both saccharin and saccharin salts. Included in the FDA Inactive Ingredients Database (oral solutions, syrups, tablets, and topical preparations). Included in nonparenteral medicines licensed in the UK. Included in the Canadian List of Acceptable Non-medicinal Ingredients.
Saccharin Preparation Products And Raw materials
Raw materials
1of2
chevron_rightPreparation Products
1of4
chevron_right| Supplier | Tel | Country | ProdList | Advantage | Inquiry |
|---|---|---|---|---|---|
| Shree Vardayini Chemical Industries Pvt Ltd | +91-8048756842 +91-7359332555 | Gujarat, India | 5 | 58 | Inquiry |
| Sunmoon Pharmaceuticals Pvt Ltd | +91-9823134983 +91-9890725812 | Maharashtra, India | 17 | 58 | Inquiry |
| Blue Jet Healthcare Ltd | +91-02222071691 +91-9082861262 | Mumbai, India | 86 | 58 | Inquiry |
| ALPHA CHEMIKA | +91-22-22061123 +91-22-66382501 | Mumbai, India | 1681 | 43 | Inquiry |
| Scientific OEM | +91-22- 2343 7546 / 2341 3094 | New Delhi, India | 1996 | 38 | Inquiry |
| dkpharmachem | 91 -251 -2690366 | New Delhi, India | 60 | 46 | Inquiry |
| Venkateshwara Life Sciences | +91 9963894542 | New Delhi, India | 88 | 50 | Inquiry |
| A.J Chemicals | 91-9810153283 | New Delhi, India | 6124 | 58 | Inquiry |
| CLEARSYNTH LABS LTD. | +91-22-45045900 | Hyderabad, India | 6351 | 58 | Inquiry |
| TCI Chemicals (India) Pvt. Ltd. | 1800 425 7889 | New Delhi, India | 6778 | 58 | Inquiry |
| Supplier | Advantage |
|---|---|
| Shree Vardayini Chemical Industries Pvt Ltd | 58 |
| Sunmoon Pharmaceuticals Pvt Ltd | 58 |
| Blue Jet Healthcare Ltd | 58 |
| ALPHA CHEMIKA | 43 |
| Scientific OEM | 38 |
| dkpharmachem | 46 |
| Venkateshwara Life Sciences | 50 |
| A.J Chemicals | 58 |
| CLEARSYNTH LABS LTD. | 58 |
| TCI Chemicals (India) Pvt. Ltd. | 58 |





