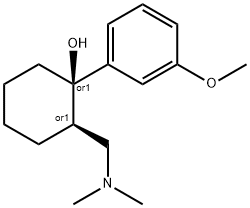
Tramadol
- CAS No.
- 27203-92-5
- Chemical Name:
- Tramadol
- Synonyms
- 2-((dimethylamino)methyl)-1-(m-methoxyphenyl)-cyclohexano;(1R,2R)-2-(Dimethylaminomethyl)-1-(3-methoxyphenyl)cyclohexan-1-ol;E 265;TRAMAL;CG 315E;CRISPIN;U 26255A;Tramadol;cis-Tramadol;Racemic tramadol
- CBNumber:
- CB7104738
- Molecular Formula:
- C16H25NO2
- Molecular Weight:
- 263.38
- MOL File:
- 27203-92-5.mol
- Modify Date:
- 2023/5/4 17:34:36
| Melting point | 178-181 °C |
|---|---|
| Boiling point | 406.62°C (rough estimate) |
| Density | 0.9903 (rough estimate) |
| refractive index | 1.4909 (estimate) |
| storage temp. | 2-8°C |
| pka | 14.47±0.40(Predicted) |
| form | solid |
| color | white to off-white |
| CAS DataBase Reference | 27203-92-5(CAS DataBase Reference) |
| NIST Chemistry Reference | Tramadol(27203-92-5) |
| EPA Substance Registry System | Cyclohexanol, 2-[(dimethylamino)methyl]-1-(3-methoxyphenyl)-, (1R,2R)-rel- (27203-92-5) |
SAFETY
Risk and Safety Statements
| Symbol(GHS) |  GHS06 |
|---|---|
| Signal word | Danger |
| Hazard statements | H301-H336 |
| Precautionary statements | P261-P271-P304+P340-P312-P403+P233-P405-P501-P264-P270-P301+P310-P321-P330-P405-P501 |
| Hazard Codes | Xn |
| Risk Statements | 22 |
| RIDADR | UN 2811 6.1/PG 3 |
| WGK Germany | 2 |
Tramadol Chemical Properties,Uses,Production
Chemical Properties
Light Yellow Oil
Uses
Tramadol is thought to produce analgesia by two distinct actions. First, it has
agonist activity at the MOP and KOP receptors. Tramadol itself is a prodrug,
with most of its analgesia mediated by a metabolite – O-desmethyltramadol –
that has a 200-fold higher affinity for the MOP receptor. I t is metabolised by
cytochrome P450 (CYP2D6 and CYP3A4), and its potency is therefore
affected by a patient's CYP genetics, with rapid and poor metabolisers.
S econd, it enhances the descending inhibitory systems in the spinal cord by inhibiting noradrenaline reuptake and releasing serotonin from nerve
endings. It is available in immediate- and sustained-release oral preparations
and for parenteral administration. I ts use is contraindicated in patients
receiving monoamine oxidase inhibitors (MAOIs). Caution must also be
exercised in hepatic impairment as its clearance is reduced to a much greater
extent than morphine and related agents.
General Description
Tramadol (Ultram) is an analgesic agent with multiple mechanismsof action. It is a weak μ-agonist with approximately30% of the analgesic effect antagonized by the opioid antagonistnaloxone. Used at recommended doses, it has minimaleffects on respiratory rate, heart rate, blood pressure, or GItransit times. Structurally, tramadol resembles codeine with the B, D, and E ring removed. The manufacturer states thatpatients allergic to codeine should not receive tramadol, becausethey may be at increased risk for anaphylactic reactions. Tramadol is synthesized and marketed as the racemicmixture of two (the [2S, 3S] [-] and the [2R, 3R] [+]) of thefour possible enantiomers. The (+) enantiomer is about30 times more potent than the (—) enantiomer; however,racemic tramadol shows improved tolerability.Neurotransmitter reuptake inhibition is also responsible forsome of the analgesic activity with the (—) enantiomer primarilyresponsible for norepinephrine reuptake and the (+)enantiomer responsible for inhibiting serotonin reuptake. Like codeine, tramadol is O-demethylated viaCYP2D6 to a more potent opioid agonist having 200-foldhigher affinity for the opioid receptor than the parent compound.Tramadol was initially marketed as nonaddictive, anda 3-year follow up study showed that the abuse potential isvery low, but not zero. Most abusers of tramadol have abusedopioid drugs in the past. Both enantiomers of tramadoland the major O-demethylated metabolite are proconvulsive,and tramadol should not be used in patients with a lowseizurethreshold including patients with epilepsy.
Mechanism of action
Fentanyl is a μ agonist with approximately 80 times greater potency than morphine. Fentanyl has been used in combination with nitrous oxide for “ balanced” anesthesia and in combination with droperidol for “ neurolepalgesia.” The advantages of fentanyl over morphine for anesthetic procedures are its shorter duration of action (1–2 hours) and the fact that it does not cause histamine release on intravenous injection.
Pharmacokinetics
The analgesic activity of tramadol is attributed to a synergistic effect caused by the opioid activity
of the (+)-isomer and the neurotransmitter reuptake blocking effect of the (–)-isomer. The
(+)-isomer possesses weak μ opioid agonist activity equivalent to approximately 1/3,800 that of
morphine. The O-desmethyl metabolite (CYP2D6) of (±)-tramadol has improved μ opioid activity
equivalent to 1/35 that of morphine. Affinity for both δ and κ receptors is improved. Despite its
higher opioid potency, the contribution of O-desmethlytramedol to the overall analgesic effect has
been questioned but not well studied. Individuals who lack CYP2D6 or are taking a CYP2D6
inhibitor have a reduced effect to tramadol. The fact that naloxone causes a decrease in the
analgesic potency of tramadol argues strongly for an opioid component to the analgesic activity.
(–)-T ramadol possesses only 1/20 the opioid activity of its (+)-isomer, but it has good activities for
inhibition of norepinephrine (Ki = 0.78 μM) and serotonin (Ki = 0.99 μM) reuptake. Tramadol's
neurotransmitter reuptake activity is approximately 1/20 that of imipramine, a tricyclic
antidepressant agent that is used widely in pain management. Although none of the individual
pharmacological activities of tramadol is impressive, they interact to give a synergistic analgesic
effect that is clinically useful.
Tramadol has been used in Europe since the 1980s and was introduced to the U.S. market in 1995.
The drug is nonaddicting and, thus, is not a scheduled agent. In addition, tramadol does not cause
respiratory depression or constipation.
Tramadol Preparation Products And Raw materials
Raw materials
Preparation Products
27203-92-5(Tramadol)Related Search:
1of4
chevron_right




