CHLOROQUINE
- CAS No.
- 54-05-7
- Chemical Name:
- CHLOROQUINE
- Synonyms
- Diphenylhydramine;Aralen;Cloroquine;Chloroquin;(7-chloro-4-(4-diethylamino-1-methylbutylamino)-quinoline;w7618;sn7618;W 7618;Imagon;win244
- CBNumber:
- CB7284322
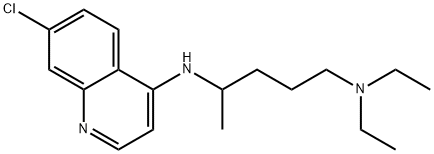
- Molecular Formula:
- C18H26ClN3
- Molecular Weight:
- 319.87
- MOL File:
- 54-05-7.mol
- Modify Date:
- 2023/5/18 11:31:08
| Melting point | 87° |
|---|---|
| Boiling point | 475.41°C (rough estimate) |
| Density | 1.0500 (rough estimate) |
| refractive index | 1.6010 (estimate) |
| storage temp. | 2-8°C(protect from light) |
| solubility | Chloroform (Slightly), Methanol (Slightly) |
| form | Solid |
| pka | pKa 8.4(H2O t = 20) (Uncertain) |
| color | White to Light Brown |
| Stability | Stable, but light sensitive. Incompatible with strong oxidizing agents. |
| IARC | 3 (Vol. 13, Sup 7) 1987 |
| EPA Substance Registry System | 1,4-Pentanediamine, N4-(7-chloro-4-quinolinyl)-N1,N1-diethyl- (54-05-7) |
SAFETY
Risk and Safety Statements
| Symbol(GHS) |  GHS07 |
|---|---|
| Signal word | Warning |
| Hazard statements | H302 |
| Precautionary statements | P264-P270-P301+P312-P330-P501 |
| Toxicity | LD50 oral in rat: 330mg/kg |
CHLOROQUINE Chemical Properties,Uses,Production
Description
Chloroquine is the most effective of the hundreds of 4-aminoquinolines synthesized and tested during World War II as potential antimalarials. Structure–activity relationships demonstrated that the chloro at the 8-position increased activity, whereas alkylation at C-3 and C-8 diminished activity. The replacement of one of its N-ethyl groups with an hydroxyethyl produced hydroxychloroquine, a compound with reduced toxicity that is rarely used today except in cases of rheumatoid arthritis.
Chemical Properties
solid
Uses
Chloroquine used in the treatment of malaria and MDR-strains. It is a COVID19-related research product.
Indications
Chloroquine (Aralen) is one of several 4-aminoquinoline derivatives that display antimalarial activity. Chloroquine is particularly effective against intraerythrocytic forms because it is concentrated within the parasitized erythrocyte. This preferential drug accumulation appears to occur as a result of specific uptake mechanisms in the parasite. Chloroquine appears to work by intercalation with DNA, inhibition of heme polymerase or by interaction with Ca++–calmodulinmediated mechanisms. It also accumulates in the parasite’s food vacuoles, where it inhibits peptide formation and phospholipases, leading to parasite death.
Definition
ChEBI: An aminoquinoline that is quinoline which is substituted at position 4 by a [5-(diethylamino)pentan-2-yl]amino group at at position 7 by chlorine. It is used for the treatment of malaria, hepatic amoebiasis, lupus erythematosus, light-sensitive skin erupti ns, and rheumatoid arthritis.
World Health Organization (WHO)
Chloroquine, a 4-aminoquinoline derivative, was introduced in the 1940s for the treatment and prophylaxis of malaria. It was subsequently found to be effective in higher and prolonged dosage in the treatment of lupus erythematosus, rheumatoid arthritis and nephritis. In the early 1970s its use in these latter conditions was largely discontinued when it was found that prolonged daily administration at high dosage was associated with cases of retinopathy resulting from local deposition of the compound. Chloroquine however remains a valuable drug. It can be used continuously at the dosages required for malaria prophylaxis for as long as five years without risk of undue accumulation and it is included in the WHO Model List of Essential Drugs for both its antimalarial and antiamoebic activity. (Reference: (WHTAC1) The Use of Essential Drugs, 2nd Report of the WHO Expert Committee, 722, , 1985)
Antimicrobial activity
Chloroquine accumulates 300-fold in infected erythrocytes and acts against the early erythrocytic stages of all four species of Plasmodium that cause human malaria. It is also active against the gametocytes of P. vivax, P. ovale and P. malariae, but not against the hepatic stages or mature erythrocytic schizonts and merozoites.
Acquired resistance
Resistance of P. falciparum is widespread and has become a major problem. The mechanism appears to be either decreased uptake or increased efflux of the drug by the parasite, or both. Changes in genes encoding a P-glycoprotein homolog, Pfmdr1, and another putative transporter, Pfcrt, are associated with resistance. Reversal of resistance with, for example, verapamil or probenecid has been demonstrated in experimental models, but human trials have been disappointing. Chloroquine-resistant P. vivax has been reported in South America and South East Asia.
Hazard
Toxic by ingestion. Questionable carcinogen.
Pharmaceutical Applications
A synthetic 4-aminoquinoline, formulated as the phosphate or sulfate for oral administration and as the hydrochloride or sulfate for parenteral use. The salts are soluble in water.
Mechanism of action
The absorption of chloroquine from the gastrointestinal tract is rapid and complete. The drug is distributed widely and is extensively bound to body tissues, with the liver containing 500 times the blood concentration. Such binding is reflected in a large volume of distribution (Vd). Desethylchloroquine is the major metabolite formed following hepatic metabolism, and both the parent compound and its metabolites are slowly eliminated by renal excretion.The half-life of the drug is 6 to 7 days.
Pharmacokinetics
Oral absorption: 80–90%
Cmax 300 mg oral: 0.25 mg/L after 1–6 h
Plasma half-life: c. 9 days (mean)
Volume of distribution: 200 L/kg
Plasma protein binding: 50–70%
There is extensive tissue binding and a high affinity for melanin-
containing tissues. Chloroquine is extensively metabolized
to a biologically active monodesethyl derivative that
forms about 20% of the plasma level of the drug. The mean
elimination half-life results from an initial phase (3–6 days),
a slow phase (12–14 days) and a terminal phase (40 days).
Renal clearance is about 50% of the dose.
Clinical Use
The drug is effective against all four types of malaria with the exception of chloroquine-resistant P. falciparum. Chloroquine destroys the blood stages of the infection and therefore ameliorates the clinical symptoms seen in P. malariae, P. vivax, P. ovale, and sensitive P. falciparum forms of malaria. The disease will return in P. vivax and P. ovale malaria, however, unless the liver stages are sequentially treated with primaquine after the administration of chloroquine. Chloroquine also can be used prophylactically in areas where resistance does not exist. In addition to its use as an antimalarial, chloroquine has been used in the treatment of rheumatoid arthritis and lupus erythematosus, extraintestinal amebiasis, and photoallergic reactions.
Side effects
Minor side effects such as dizziness, headache, rashes, nausea and diarrhea are common. Pruritus occurs in up to 20% of Africans taking chloroquine. Long-term treatment can induce CNS effects and cumulative dosing over many years may cause retinopathy. Rarely, photosensitization, tinnitus and deafness have occurred.
Environmental Fate
The exact mechanism of action of CQ and HCQ is not completely understood but involves inhibition of DNA and RNA polymerase. They are also direct myocardial depressants that impair cardiac conduction through membrane stabilization. It is unclear how they work in autoimmune diseases.
CHLOROQUINE Preparation Products And Raw materials
Raw materials
Preparation Products
| Supplier | Tel | Country | ProdList | Advantage | Inquiry |
|---|---|---|---|---|---|
| CLEARSYNTH LABS LTD. | +91-22-45045900 | Hyderabad, India | 6351 | 58 | Inquiry |
| A.J Chemicals | 91-9810153283 | New Delhi, India | 6124 | 58 | Inquiry |
| Triveni chemicals | 08048762458 | New Delhi, India | 6093 | 58 | Inquiry |
| Medinn Agencies | 08047647335 | Delhi, India | 19 | 58 | Inquiry |
| Jocund India Limited | 09868450067 | Delhi, India | 2 | 58 | Inquiry |
| Chandra Prabhu Pharma Services | 08048372381Ext 506 | Maharashtra, India | 19 | 58 | Inquiry |
| AYA NUTRITIONS | 08048987442 | Uttar Pradesh, India | 1 | 58 | Inquiry |
| Pharma Affiliates | 172-5066494 | Haryana, India | 6761 | 58 | Inquiry |
| Pharmaffiliates Analytics and Synthetics P. Ltd | +91-172-5066494 | Haryana, India | 6773 | 58 | Inquiry |
| SHANDONG ZHI SHANG CHEMICAL CO.LTD | +86 18953170293 | China | 2931 | 58 | Inquiry |
| Supplier | Advantage |
|---|---|
| CLEARSYNTH LABS LTD. | 58 |
| A.J Chemicals | 58 |
| Triveni chemicals | 58 |
| Medinn Agencies | 58 |
| Jocund India Limited | 58 |
| Chandra Prabhu Pharma Services | 58 |
| AYA NUTRITIONS | 58 |
| Pharma Affiliates | 58 |
| Pharmaffiliates Analytics and Synthetics P. Ltd | 58 |
| SHANDONG ZHI SHANG CHEMICAL CO.LTD | 58 |
Related articles
- Toxicity of action of Chloroquine
- The molecular formula of chloroquine is C18H26ClN3 and its molecular weight is 320. Chloroquine (Aralen) is formulated as tabl....
- Apr 1,2022
- Mechanism of Chloroquine
- Chloroquine (CQ) and hydroxychloroquine (HCQ) are synthetic 4-aminoquinolines. CQ has been used as an antimalarial drug since ....
- Jan 7,2022
- Chloroquine
- Chloroquine is an aminoquinoline used for the prevention and therapy of malaria. It is also effective in extraintestinal amebi....
- Nov 10,2021
54-05-7(CHLOROQUINE)Related Search:
1of4
chevron_right




