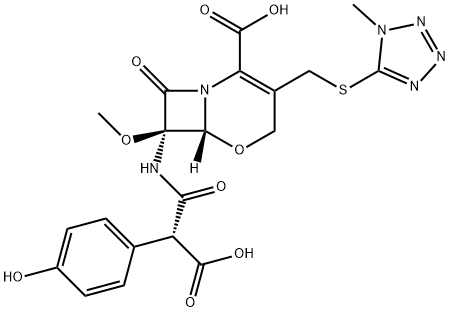
Latamoxef
- CAS No.
- 64952-97-2
- Chemical Name:
- Latamoxef
- Synonyms
- LMOX;C07231;latamoxef;Oxa-cephem;lamoxactam;Latamoxef Acid;Latamoxef USP/EP/BP;Listeria MOX Supplement, Moxalactam;4-Isoxazolecarboxylicacid,5-hydroxy-8-methyl-,ethylester;5-oxa-1-azabicyclo(4.2.0)oct-2-ene-2-carboxylicacid,7-((carboxy(4-hydroxyphen
- CBNumber:
- CB7497197
- Molecular Formula:
- C20H20N6O9S
- Molecular Weight:
- 520.47
- MOL File:
- 64952-97-2.mol
- Modify Date:
- 2022/12/21 16:56:50
Latamoxef Chemical Properties,Uses,Production
Uses
Latamoxef is an oxacephem antibiotic usually grouped with the cephalosporins. An anti-bacterial agent.
Definition
ChEBI: A broad-spectrum oxacephem antibiotic in which the oxazine ring is substituted with a tetrazolylthiomethyl group and the azetidinone ring carries methoxy and 2-carboxy-2-(4-hydroxyphenyl)acetamido substituents.
World Health Organization (WHO)
Latamoxef, a cefamycin antibiotic, was introduced in 1982 for the treatment of serious infections. Its use has subsequently been associated with reports of clinically important haemorrhage, sometimes fatal, and in some countries routine co-administration of vitamin K is advised to minimize this risk.
Antimicrobial activity
Moxalactam. A semisynthetic 7-methoxyoxacephem, supplied
as the disodium salt.It is generally slightly
less active than cefotaxime, especially against Staph. aureus,
but unlike other group 4 cephalosporins it exhibits fairly good
activity against B. fragilis. Other Bacteroides spp. are generally
less susceptible. The 7-methoxy substitution, also found in
cephamycins such as cefoxitin, confers resistance to hydrolysis
by a wide range of β-lactamases including those of Staph.
aureus, various enterobacteria and B. fragilis. Resistance, predominantly
in Enterobacter spp., Ps. aeruginosa and Ser. marcescens
due to induction of chromosomal enzymes, has
been found in vitro and in some patients.
A 500 mg intramuscular injection achieves a serum concentration
of 12–22 mg/L after 1.2 h. Infusion of 1 g over
30 min results in a concentration of 60 mg/L. The plasma
half-life is c. 2 h and plasma protein binding 40–50%. There is reasonably good penetration into serous fluids, the concentration
in ascitic fluid reaching 75% and in pleural fluid 50%
of the concentration simultaneously present in the serum.
Levels of 5–35 mg/L have been obtained in inflamed meninges.
Sputum levels are of the order of 2 mg/L following 1 g of
the drug intravenously.
Renal elimination accounts for 90% of the clearance, but
significant concentrations are found in the feces. Excretion is
depressed in renal failure. Hemodialysis removes 48–51% of
the drug in 4 h; peritoneal dialysis has little or no effect.
Increased bleeding and decreases in platelet function associated
with the methylthiotetrazole side chain are sufficiently
common to have been cited as reasons for restricting use of
the agent. Use is contraindicated in patients on anticoagulant
therapy. Uses are similar to those of group 4 cephalosporins.
It is generally less successful in the treatment of infections due
to Gram-positive organisms.
Latamoxef Preparation Products And Raw materials
| Supplier | Tel | Country | ProdList | Advantage | Inquiry |
|---|---|---|---|---|---|
| Pharma Affiliates | 172-5066494 | Haryana, India | 6761 | 58 | Inquiry |
| Pharmaffiliates Analytics and Synthetics P. Ltd | +91-172-5066494 | Haryana, India | 6773 | 58 | Inquiry |
| career henan chemical co | +86-0371-86658258 +8613203830695 | China | 29898 | 58 | Inquiry |
| Chongqing Chemdad Co., Ltd | +86-023-6139-8061 +86-86-13650506873 | China | 39916 | 58 | Inquiry |
| CONIER CHEM AND PHARMA LIMITED | +8618523575427 | China | 49391 | 58 | Inquiry |
| TargetMol Chemicals Inc. | +1-781-999-5354 +1-00000000000 | United States | 19892 | 58 | Inquiry |
| Shaanxi Dideu Medichem Co. Ltd | +86-029-89586680 +86-18192503167 | China | 9030 | 58 | Inquiry |
| changzhou huayang technology co., ltd | +8615250961469 | China | 9827 | 58 | Inquiry |
| Hefei TNJ Chemical Industry Co.,Ltd. | 0551-65418684 +8618949823763 | China | 25363 | 58 | Inquiry |
| GIHI CHEMICALS CO.,LIMITED | +8618058761490 | China | 49999 | 58 | Inquiry |





