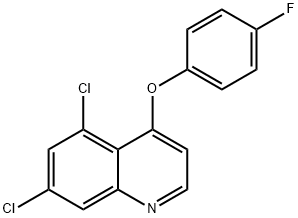
QUINOXYFEN
- CAS No.
- 124495-18-7
- Chemical Name:
- QUINOXYFEN
- Synonyms
- Quinoxyphen;Quintec;QUINOXYFEN;FORTRESS(R);Legend Elios;Quinoxyfen 0.1;Quinoxyfen >CAS:124495-18-7;quinoxyfen (ISO);Legend (fungicide)
- CBNumber:
- CB7757516
- Molecular Formula:
- C15H8Cl2FNO
- Molecular Weight:
- 308.13
- MOL File:
- 124495-18-7.mol
- MSDS File:
- SDS
- Modify Date:
- 2025/1/27 9:38:02
| Melting point | 105-106° |
|---|---|
| Boiling point | 423℃ |
| Density | 1.430 |
| vapor pressure | 1.2 x 10-5 Pa (20 °C) |
| Flash point | >100 °C |
| storage temp. | 0-6°C |
| solubility | Chloroform (Slightly), Methanol (Slightly) |
| form | Solid |
| Water Solubility | 0.12 mg l-1 (20 °C) |
| pka | 2.87±0.50(Predicted) |
| color | White to Light yellow |
| Merck | 14,8079 |
| LogP | 5.1 at 20℃ |
| Dissociation constant | 3.56 |
| EPA Substance Registry System | Quinoxyfen (124495-18-7) |
SAFETY
Risk and Safety Statements
| Symbol(GHS) |   GHS07,GHS09 |
|---|---|
| Signal word | Warning |
| Hazard statements | H317-H410 |
| Precautionary statements | P261-P272-P273-P280-P302+P352-P333+P313 |
| Hazard Codes | Xi,N |
| Risk Statements | 43-50/53 |
| Safety Statements | 24-37-46-60-61 |
| RIDADR | UN3077 9/PG 3 |
| WGK Germany | 3 |
| RTECS | VB4287500 |
| HazardClass | 9 |
| PackingGroup | III |
| HS Code | 38220090 |
| Hazardous Substances Data | 124495-18-7(Hazardous Substances Data) |
| Toxicity | LD50 orally in rats: >5000 mg/kg; dermally in rabbits: >2000 mg/kg; by inhalation in rats: >3.38 mg/l (Longhurst) |
QUINOXYFEN Chemical Properties,Uses,Production
Chemical Properties
Off-White Solid
Uses
Quinoxyfen is under development for the control of powdery mildew in cereals and grapes.
Definition
ChEBI: A member of the class of quinolines carrying two chloro substituents at positions 5 and 7 together with a 4-fluorophenoxy substituent at position 4. A fungicide used mainly to control powdery mildew in cereals.
Environmental Fate
The stability of quinoxyfen in the soil has been shown
to vary depending on soil type and source. DT50 values
obtained in the field varied from 5 to 454 days for a
range of soil types. The strong adsorptive properties of
quinoxyfen reduce its soil dissipation rate, but result in
no leaching potential of this fungicide into waterways or
groundwater. The primary metabolite formed in the soil is
3-hydroxyquinoxyfen . A secondary soil metabolite
is 5,7-dichloro-4-hydroxyquinoline (DCHQ). DCHQ was
also found not to leach, even in sandy soils. Under acidic
aqueous conditions, DCHQ was the primary metabolite
found, and this was produced in greater quantities at
acidic pHs. An additional metabolite was isolated from
both water and the sediment in an aqueous clay loam
system. Although not positively identified, it is suspected
to be 6-hydroxyquinoxyfen.
A similar hydrolysis profile is observed in water
as in soil (3). The primary product produced under
acidic conditions in the absence of light was DCHQ.
However, in the presence of light, photolysis was greatly
increased and dose dependent on the amount of sunlight
received. The primary photolysis product was 2-chloro-10-
fluoro[1]benzopyrano[2,3,4-de]quinoline (CFBPQ).
Metabolic pathway
Quinoxyfen is a novel fungicide for the control of powdery mildew in
cereals. Its mode of action is unknown but it appears to differ from those
of current fungicides and thus may be novel (Longhurst et al., 1996).
Quinoxyfen is tightly bound to soil components and is somewhat persistent
in this medium but in aqueous solution it is subject to rapid
photodecomposition. Photodegradation is therefore likely to be an important
process in its immediate removal from the environment. Plant
metabolites have not been reported but unchanged quinoxyfen has been
confirmed as the major residue.
The compound is rapidly metabolised and eliminated following ingestion
by rats and goats. Metabolism involves mainly hydroxylation of the
intact quinoxyfen, cleavage at the ether bond and conjugation of the
resulting metabolites. The information presented below was obtained
from two sources (DowElanco, 1996; Reeves et al., 1996).
QUINOXYFEN Preparation Products And Raw materials
Raw materials
Preparation Products
| Supplier | Tel | Country | ProdList | Advantage | Inquiry |
|---|---|---|---|---|---|
| CLEARSYNTH LABS LTD. | +91-22-45045900 | Hyderabad, India | 6257 | 58 | Inquiry |
| A.J Chemicals | 91-9810153283 | New Delhi, India | 6100 | 58 | Inquiry |
| TCI Chemicals (India) Pvt. Ltd. | 1800 425 7889 | New Delhi, India | 6768 | 58 | Inquiry |
| Clearsynth Labs | 91-22-45045900 | Maharashtra, India | 3887 | 58 | Inquiry |
| Vyshno Bio Sciences | 91-7095439993 | Hyderabad, India | 239 | 58 | Inquiry |
| ATK CHEMICAL COMPANY LIMITED | +undefined-21-51877795 | China | 33024 | 60 | Inquiry |
| Zhejiang ZETian Fine Chemicals Co. LTD | +8618957127338 | China | 2136 | 58 | Inquiry |
| SIMAGCHEM CORP | +86-13806087780 | China | 17357 | 58 | Inquiry |
| TargetMol Chemicals Inc. | +1-781-999-5354 +1-00000000000 | United States | 32222 | 58 | Inquiry |
| AFINE CHEMICALS LIMITED | +86-0571-85134551 | China | 15417 | 58 | Inquiry |
| Supplier | Advantage |
|---|---|
| CLEARSYNTH LABS LTD. | 58 |
| A.J Chemicals | 58 |
| TCI Chemicals (India) Pvt. Ltd. | 58 |
| Clearsynth Labs | 58 |
| Vyshno Bio Sciences | 58 |
| ATK CHEMICAL COMPANY LIMITED | 60 |
| Zhejiang ZETian Fine Chemicals Co. LTD | 58 |
| SIMAGCHEM CORP | 58 |
| TargetMol Chemicals Inc. | 58 |
| AFINE CHEMICALS LIMITED | 58 |





