MERCURIC BROMIDE
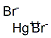
- CAS No.
- 7789-47-1
- Chemical Name:
- MERCURIC BROMIDE
- Synonyms
- HgBr2;dibromomercury;MERCURY BROMIDE;mercurydibromide;MERCURIC BROMIDE;mecury dibromide;mercuricdibromide;merciuric bromide;MercuricBromideGr;Mercury(Ⅱ)bromide
- CBNumber:
- CB7759000
- Molecular Formula:
- Br2Hg
- Molecular Weight:
- 360.4
- MOL File:
- 7789-47-1.mol
- Modify Date:
- 2024/7/9 8:40:47
| Melting point | 236 °C(lit.) |
|---|---|
| Boiling point | 322 °C(lit.) |
| Density | 6.1 |
| vapor pressure | 1 mm Hg ( 136.5 °C) |
| Flash point | 322-325°C subl. |
| storage temp. | Poison room |
| solubility | Soluble in hot alcohol, methanol, HCl, HBr |
| form | beads |
| Specific Gravity | 6.109 |
| color | White |
| Water Solubility | g/100g solution H2O: 0.3 (0°C), 0.611±0.002 (25°C), 4.7 (100°C) [KRU93]; very soluble hot alcohol, methanol, HCl, HBr; slightly soluble chloroform [MER06] |
| Sensitive | Light Sensitive |
| Merck | 14,5875 |
| Solubility Product Constant (Ksp) | pKsp: 19.21 |
| Stability | Stable, but may be light sensitive. Incompatible with potassium and sodium. |
| CAS DataBase Reference | 7789-47-1(CAS DataBase Reference) |
| EPA Substance Registry System | Mercury bromide (HgBr2) (7789-47-1) |
SAFETY
Risk and Safety Statements
| Symbol(GHS) |    GHS06,GHS08,GHS09 |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Signal word | Danger | |||||||||
| Hazard statements | H300+H310+H330-H373-H410 | |||||||||
| Precautionary statements | P262-P264-P273-P280-P302+P352+P310-P304+P340+P310 | |||||||||
| Hazard Codes | T+,N | |||||||||
| Risk Statements | 26/27/28-33-50/53 | |||||||||
| Safety Statements | 13-28-45-60-61 | |||||||||
| RIDADR | UN 1634 6.1/PG 2 | |||||||||
| WGK Germany | 3 | |||||||||
| RTECS | OV7415000 | |||||||||
| F | 8 | |||||||||
| TSCA | Yes | |||||||||
| HazardClass | 6.1 | |||||||||
| PackingGroup | II | |||||||||
| HS Code | 28521000 | |||||||||
| NFPA 704 |
|
MERCURIC BROMIDE price More Price(12)
| Manufacturer | Product number | Product description | CAS number | Packaging | Price | Updated | Buy |
|---|---|---|---|---|---|---|---|
| Sigma-Aldrich | 200085 | Mercury(II) bromide ACS reagent | 7789-47-1 | 100G | ₹16713.8 | 2022-06-14 | Buy |
| Sigma-Aldrich(India) | 200085 | Mercury(II) bromide ACS reagent | 7789-47-1 | 100G | ₹16713.8 | 2022-06-14 | Buy |
| Sigma-Aldrich | 1.04421 | Mercury(II) bromide for analysis EMSURE? ACS,Reag. Ph Eur | 7789-47-1 | 50G | ₹44100 | 2022-06-14 | Buy |
| Sigma-Aldrich(India) | 1.04421 | Mercury(II) bromide for analysis EMSURE? ACS,Reag. Ph Eur | 7789-47-1 | 50G | ₹44100 | 2022-06-14 | Buy |
| ALFA India | ALF-044816-22 | Mercury(II) bromide, ACS | 7789-47-1 | 100g | ₹8741 | 2022-05-26 | Buy |
MERCURIC BROMIDE Chemical Properties,Uses,Production
Chemical Properties
Mercuric Bromide is a crystalline solid
Uses
Medicine.
General Description
White rhombic crystals. Sensitive to light. Slightly soluble in water and denser than water. Severely toxic by inhalation and ingestion.
Air & Water Reactions
Slightly soluble in water.
Reactivity Profile
MERCURIC BROMIDE is incompatible with acetylene, ammonia, chlorine dioxide, azides, calcium (amalgam formation), sodium carbide, lithium, rubidium, copper . Reacts with sodium azide to give mercury(II) azide, which is sensitive to shock, friction, and heat. Mixing with hydrazine salts in basic solution produced a heat or shock sensitive yellow precipitate [Annalen, 1899, 305, 191]. Reacts violently with chlorine trifluoride *with ignition often occurring.
Hazard
Toxic by inhalation, ingestion, and skin absorption; strong irritant.
Health Hazard
TOXIC; inhalation, ingestion or skin contact with material may cause severe injury or death. Contact with molten substance may cause severe burns to skin and eyes. Avoid any skin contact. Effects of contact or inhalation may be delayed. Fire may produce irritating, corrosive and/or toxic gases. Runoff from fire control or dilution water may be corrosive and/or toxic and cause pollution.
Fire Hazard
Non-combustible, substance itself does not burn but may decompose upon heating to produce corrosive and/or toxic fumes. Some are oxidizers and may ignite combustibles (wood, paper, oil, clothing, etc.). Contact with metals may evolve flammable hydrogen gas. Containers may explode when heated.
Safety Profile
A poison by ingestion, skin contact, and intraperitoneal routes. Vigorous reaction with indium at 35OC. Incompatible with sodmm and potassium. When heated to decomposition it emits very toxic fumes of Brand Hg. See also MERCURY COMPOUNDS and BROMIDES.
Synthesis
Five parts of Hg are covered with 60 parts of H2O and allowed
to react at 50 °C (vigorous stirring) with four parts of Br2 , which
is added dropwise as long as no permanent color is formed. The
solution is then brought to a boil, filtered hot, and placed in an ice
bath to induce crystallization. The salt is dried at as low a
temperature as possible. Purification is by careful double or triple
sublimation from a porcelain dish heated on a sand bath and
covered with a Petri dish. When very high purity is required
(e.g., for conductivity measurements), it may be necessary to re -
peat the sublimation several times more.
Hg + Br2 = HgBr2
Potential Exposure
This compound has applications in medicine.
Shipping
UN1634 Mercuric bromides, Hazard Class: 6.1; Labels: 6.1-Poisonous materials
Purification Methods
Crystallise it from hot saturated ethanolic solution, dry and keep it at 100o for several hours under a vacuum, then sublime it. [Garrett J Am Chem Soc 61 2744 1939.] Its solubility in H2O is 0.6% at 20o, and 22% at 100o; in EtOH it is 30% at 25o; and in MeOH it is 69.6% at 25o. [Wagenknecht & Juza Handbook of Preparative Inorganic Chemistry (Ed. Brauer) Academic Press Vol II p 1109 1965.] POISONOUS.
Incompatibilities
Violent reaction with active metals; potassium, sodium. Store away from heat and light
MERCURIC BROMIDE Preparation Products And Raw materials
7789-47-1(MERCURIC BROMIDE)Related Search:
1of4
chevron_right




