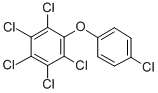
Hexachlorodiphenyloxide
- CAS No.
- 31242-93-0
- Chemical Name:
- Hexachlorodiphenyloxide
- Synonyms
- Chlorinatedphenylether;Hexachlorodiphenyloxide;CHLORINATEDDIPHENYLOXIDE;Di(trichlorophenyl) ether;O-CHLORINATEDDIPHENYLOXIDE
- CBNumber:
- CB7852020
- Molecular Formula:
- C12H4Cl6O
- Molecular Weight:
- 376.87756
- MOL File:
- 31242-93-0.mol
- Modify Date:
- 2024/12/18 14:08:57
| Density | 1.6245 (estimate) |
|---|---|
| EPA Substance Registry System | Phenyl ether, hexachloro deriv. (31242-93-0) |
Hexachlorodiphenyloxide Chemical Properties,Uses,Production
Chemical Properties
Light-yellow, very viscous liquid. Soluble in methanol and ether; very slightly soluble in water. Nonflammable.
Uses
Solvent, intermediate.
Hazard
Toxic by ingestion.
Safety Profile
Moderately toxic by ingestion and probably by inhalation. Combustible when exposed to heat, flame, or oxidizing materials. To fight fire, use water spray, fog, foam, dry chemical, CO2. When heated to decomposition it emits toxic fumes of Cl-. See also ETHERS and ALDRIN (a closely related compound).
Potential Exposure
These materials are used as dielectric fluids in the electrical industry; they may be used as organic intermediates to make drugs and other chemicals; and in dry cleaning detergents. Used in the manufacture of flame-inhibiting polymers, as corrosion inhibitors, drycleaning detergents, thermal lubricants, additives for soaps and lotions, and in the manufacture of hydraulic fluids, pesticides, wood preservatives, and electric insulators.
Shipping
UN3082 Environmentally hazardous substances, liquid, n.o.s., Hazard class: 9; Labels: 9-Miscellaneous hazardous material, Technical Name Required. UN3077 Environmentally hazardous substances, solid, n.o.s., Hazard class: 9; Labels: 9-Miscellaneous hazardous material, Technical Name Required.
Incompatibilities
Incompatible with oxidizers (chlorates, nitrates, peroxides, permanganates, perchlorates, chlorine, bromine, fluorine, etc.); contact may cause fires or explosions. Keep away from alkaline materials, strong bases, strong acids, oxoacids, epoxides. May be able to form unstable peroxides. Reacts with aluminum.
Waste Disposal
For trichloro phenyl ether, solution in a flammable solvent and incineration in a furnace with afterburner and scrubber is recommended.





