Chromium
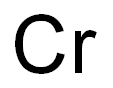
- CAS No.
- 7440-47-3
- Chemical Name:
- Chromium
- Synonyms
- chrome;chrom;CHROMIUM POWDER;2-5% Nitric acid;YEAST BOUND CHROMIUM;CR000070;CR007930;CR000025;CR000060;CR004850
- CBNumber:
- CB7854190
- Molecular Formula:
- Cr
- Molecular Weight:
- 52
- MOL File:
- 7440-47-3.mol
- MSDS File:
- SDS
- Modify Date:
- 2025/3/12 7:03:40
| Melting point | 1857 °C (lit.) |
|---|---|
| Boiling point | 2672 °C (lit.) |
| Density | 7.14 g/mL at 25 °C (lit.) |
| Flash point | 50 °F |
| storage temp. | no restrictions. |
| solubility | reacts with dilute acid solutions |
| form | powder |
| color | Silver-gray |
| Specific Gravity | 7.2 |
| PH | <1 (H2O, 20°C) |
| Odor | Odorless |
| Flame Color | Silvery white |
| Resistivity | 12.7 μΩ-cm, 20°C |
| Water Solubility | Insoluble in water. |
| Merck | 13,2252 |
| Exposure limits | TLV-TWA: chromium metal 0.5 mg/m3 (ACGIH and MSHA), 1 mg/m3 (OSHA); Cr(II) and Cr(III) compounds 0.5 mg/m3 (ACGIH); Cr(VI) compounds, water soluble and certain water insoluble, 0.05 mg/m3 (ACGIH). |
| Stability | Stable. Incompatible with carbonates, strong bases, mineral acids, lithium, sulfur dioxide, strong acids. |
| CAS DataBase Reference | 7440-47-3(CAS DataBase Reference) |
| IARC | 3 (Vol. Sup 7, 49) 1990 |
| NIST Chemistry Reference | Chromium(7440-47-3) |
| EPA Substance Registry System | Chromium (7440-47-3) |
| Modulus of Elasticity | 248 GPa |
|---|---|
| Hardness, Vickers | 131, Converted from Brinell |
| Hardness, Brinell | 125 |
| Hardness, Rockwell B | 72, Converted from Brinell |
| Hardness, Knoop | 144, Converted from Brinell |
SAFETY
Risk and Safety Statements
| Symbol(GHS) |    GHS02,GHS08,GHS09 |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Signal word | Warning | |||||||||
| Hazard statements | H410-H351-H228 | |||||||||
| Precautionary statements | P201-P202-P280-P308+P313-P405-P501a-P210-P240 | |||||||||
| Hazard Codes | F,C,Xn,Xi | |||||||||
| Risk Statements | 11-20/21/22-34-40-23-67-36 | |||||||||
| Safety Statements | 16-26-36/37/39-45-36/37-27 | |||||||||
| OEB | C | |||||||||
| OEL | TWA: 0.5 mg/m3 | |||||||||
| RIDADR | UN 2924 3/PG 2 | |||||||||
| WGK Germany | 3 | |||||||||
| RTECS | GB4200000 | |||||||||
| Autoignition Temperature | 580°C | |||||||||
| TSCA | Yes | |||||||||
| HS Code | 8112 21 90 | |||||||||
| HazardClass | 4.1 | |||||||||
| PackingGroup | III | |||||||||
| Hazardous Substances Data | 7440-47-3(Hazardous Substances Data) | |||||||||
| Toxicity | Elemental chromium and certain chromium compounds have been designated as carcinogens, hazardous substances, hazardous waste constituents, and priority toxic pollutants. Some of those compounds designated as hazardous are chromic acetate, chromic acid, chromic sulfate, and chromous chloride. Although chromium in the 6+ state is regarded as being the most carcinogenic, there are 6+ compounds that appear to be non-carcinogenic. In addition to their possible carcinogenicity, chromium compounds may have local allergic effects leading to dermatitis. Systemically, 6+ chromium compounds are irritants to the respiratory system and may give rise to pulmonary edema. | |||||||||
| IDLA | 250 mg Cr/m3 | |||||||||
| NFPA 704 |
|
Chromium price More Price(83)
| Manufacturer | Product number | Product description | CAS number | Packaging | Price | Updated | Buy |
|---|---|---|---|---|---|---|---|
| Sigma-Aldrich | 374849 | Chromium chips, 99.995% trace metals basis | 7440-47-3 | 50G | ₹14407.8 | 2022-06-14 | Buy |
| Sigma-Aldrich(India) | 374849 | Chromium chips, 99.995% trace metals basis | 7440-47-3 | 50G | ₹14407.8 | 2022-06-14 | Buy |
| Sigma-Aldrich(India) | 749052 | Chromium sputtering target, diam. × thickness 3.00?in. × 0.125?in., 99.95% trace metals basis | 7440-47-3 | 1EA | ₹46897.5 | 2022-06-14 | Buy |
| Sigma-Aldrich | 374849 | Chromium chips, 99.995% trace metals basis | 7440-47-3 | 250G | ₹38195.1 | 2022-06-14 | Buy |
| Sigma-Aldrich(India) | 374849 | Chromium chips, 99.995% trace metals basis | 7440-47-3 | 250G | ₹38195.1 | 2022-06-14 | Buy |
Chromium Chemical Properties,Uses,Production
Description
Chromium as a metallic element was first discovered over 200 years ago, in 1797. But the history of chromium really began several decades before this. In 1761, in the Beresof Mines of the Ural Mountains, Johann Gottlob Lehmann obtained samples of an orange-red mineral, which he called ‘Siberian red lead.’ He analyzed this mineral in 1766 and discovered that it contained lead “mineralized with a selenitic spar and iron particles.” The mineral he found was crocoite, a lead chromate (PbCrO4).
Chemical Properties
Chromium may exist in one of three valence states in compounds, , , and . The most stable oxidation state is trivalent chromium; Hexavalent chromium is a less stable state. Chromium (element) blue-white to steel-gray, lustrous, brittle, hard, odorless solid. Elemental:
Physical properties
Chromium is a silvery white/gray, hard, brittle noncorrosive metal that has chemical andphysical properties similar to the two preceding elements in period 4 (V and Ti). As one of thetransition elements, its uses its M shell rather than its outer N shell for valence electrons whencombining with other elements. Its melting point is 1,857°C, its boiling point is 2,672°C,and its density is 7.19 g/cm3.
Isotopes
There are 26 isotopes of the element chromium; four are stable and foundin nature, and the rest are artificially produced with half-lives from a few microsecondsto a few days. The four stable isotopes and their percentage of contribution to thetotal amount of chromium on Earth are as follows: 50Cr = 4.345%, 52Cr = 83.789%,53Cr = 9.501%, and 54Cr = 2.365%. Cr-50 is radioactive but has such a long halflife—1.8×10+17 years—that it is considered to contribute about 4% to the total amount ofchromium found on Earth.
Origin of Name
From the Greek word chroma or chromos, meaning “color,” because of the many colors of its minerals and compounds.
Occurrence
Chromium is the 21st most common element found in the Earth’s crust, and chromiumoxide (Cr2O3) is the 10th most abundant of the oxide compounds found on Earth. It is notfound in a free metallic state.The first source of chromium was found in the mineral crocoite. Today it is obtained fromthe mineral chromite (FeCr2O4), which is found in Cuba, Zimbabwe, South Africa, Turkey,Russia, and the Philippines. Chromite is an ordinary blackish substance that was ignored formany years. There are different grades and forms of chromium ores and compounds, based onthe classification of use of the element. Most oxides of chromium are found mixed with othermetals, such as iron, magnesium, or aluminum.Astronauts found that the moon’s basalt rocks contain several times more chromium thanis found in basalt rocks of Earth.
Characteristics
Chromium is a hard, brittle metal that, with difficulty, can be forged, rolled, and drawn,unless it is in a very pure form, in which case the chromium is easier to work with. It is anexcellent alloying metal with iron. Its bright, silvery property makes it an appropriate metal toprovide a reflective, non-corrosive attractive finish for electroplating.Various compounds of chromium exhibit vivid colors, such as red, chrome green, andchromate yellow, all used as pigments.
Uses
Chromium is used in the manufacture ofits alloys, such as chrome-steel or chromenickel-steel. It is also used for chromeplatingof other metals, for tanning leather,and in catalysts. It occurs in chromite ores(FeO·Cr2O3).
Production Methods
Chromium metal is prepared by reducing the ore in a blast furnace with carbon (coke) or silicon to form an alloy of chromium and iron called ferrochrome, which is used as the starting material for the many iron-containing alloys that employ chromium. Chromium to be used in iron-free alloys is obtained by reduction or electrolysis of chromium compounds.Chromiumisdif?culttoworkinthepuremetalform; it is brittle at low temperatures, and its high melting point makes it dif?cult to cast.
Definition
chromium: Symbol Cr. A hard silverytransition element; a.n. 24;r.a.m. 52.00; r.d. 7.19; m.p. 1857°C;b.p. 2672°C. The main ore ischromite (FeCr2O4). The metal has abody-centred-cubic structure. It is extractedby heating chromite withsodium chromate, from whichchromium can be obtained by electrolysis.Alternatively, chromite can be heated with carbon in an electricfurnace to give ferrochrome, whichis used in making alloy steels. Themetal is also used as a shiny decorativeelectroplated coating and in themanufacture of certain chromiumcompounds.
At normal temperatures the metalis corrosion-resistant. It reacts withdilute hydrochloric and sulphuricacids to give chromium(II) salts.These readily oxidize to the more stablechromium(III) salts. Chromiumalso forms compounds with the +6oxidation state, as in chromates,which contain the CrO42- ion. The elementwas discovered in 1797 byVauquelin.
General Description
Very hard gray solid with a metallic luster.
Air & Water Reactions
May be pyrophoric, as dust. Insoluble in water.
Reactivity Profile
Chromium reacts violently with NH4NO3, N2O2, Li, NO, KClO3, SO2 . Metal dusts when suspended in atmospheres of carbon dioxide may ignite and explode.
Hazard
Hexavalent chromium compounds are questionable carcinogens and corrosive on tissue, resulting in ulcers and dermatitis on prolonged contact.
Fire Hazard
Non-combustible, substance itself does not burn but may decompose upon heating to produce corrosive and/or toxic fumes. Some are oxidizers and may ignite combustibles (wood, paper, oil, clothing, etc.). Contact with metals may evolve flammable hydrogen gas. Containers may explode when heated.
Potential Exposure
Chromium metal is used in stainless and other alloy steels to impart resistance to corrosion, oxidation, and for greatly increasing the durability of metals; for chrome plating of other metals.
Carcinogenicity
Exposure to chromium compounds over a prolonged period has been observed in manyepidemiologicalstudiestoenhancetheriskofcancerof the respiratory organs among the exposed. The relationshipbetweenemploymentinindustriesproducingchromium compounds from chromite ore and enhanced risk of lungcancer iswell established.There isagreement inseveral studies that long-term exposure to some chromium-based pigments enhance the risk of lung cancer. An association has alsobeenobservedbetweenexposuretochromicacidinhard plating and lung cancer, but that association is not strong. Somestudieshaveweaklyindicatedexcessesofcancerofthe GItract,buttheresultsareinconsistentandarenotcon?rmed inwell-designedstudies.Thereisnoindicationthatchromite ore does have an associated enhanced risk of cancer. Although it has not yet been identi?ed which chromium compound (or compounds) is (are) responsible for enhanced risk of cancer in respiratory organs, there is general agreementthatitisthechromium(6+)speciesthatareresponsible for the elevated cancer risks and that the chromium species are not.
Environmental Fate
Chromium is distributed to the air, water, and soil from natural and anthropogenic sources. The environmental fate of chromium is dependent on the oxidation state and solubility of the compound and the environmental conditions affecting reduction or oxidation, such as pH. Oxidizing conditions favor the formation of Cr(VI) compounds, particularly at higher temperatures, while reducing conditions favor the formation of Cr(III) compounds. Chemical manufacturing and natural gas, oil, and gas combustion are the primary sources of chromium in the atmosphere.Most of the chromium in air eventually ends up in water or soil. Electroplating, textile manufacturing, cooling water, and leather tanning are major sources of chromium in wastewater discharges to surface waters.
Chromium(III) is the predominant oxidation state of chromium in many soils. Cr(III) binds to soil and has low mobility. A lower soil pH favors the reduction of Cr(VI) to Cr(III). Runoff from soil and industrial processes may transport chromium to surface water.Cr(VI) compounds may leach into groundwater. The pH of the soil and aquatic environment is an important factor in chromium mobility, bioavailability, and toxicity. The chromate form predominates in most natural surface waters that are basic or neutral. The hydrochromate concentration increases in more acidic conditions.
Shipping
UN3089 Metal powders, flammable, n.o.s., Hazard Class: 4.1; Labels: 4.1-Flammable solid. UN1759 Corrosive solids, n.o.s., Hazard class: 8; Labels: 8-Corrosive material, Technical Name required
Structure and conformation
Two types of chromium crystals, α and β, are obtained depending on the growth method. The β type is semi-stable. It changes to a type above 800℃. The space lattice of β-Cr belongs to the hexagonal system, and its closely-packed hexagonal lattice has lattice constants of a=0.272 nm and c=0.442 nm. The space lattice of α-Cr belongs to the cubic system, and its body-centered cubic lattice has a lattice constant of a=0.28796 nm (18℃).
Incompatibilities
Dust may be pyrophoric in air. Chromium metal (especially in finely divided or powder form) and insoluble salts reacts violently with strong oxidants, such as hydrogen peroxide, causing fire and explosion hazard. Reacts with diluted hydrochloric and sulfuric acids. Incompatible with alkalis and alkali carbonates
Waste Disposal
Recovery and recycling is a viable alternative to disposal for chromium in plating wastes; tannery wastes; cooling tower blowdown water and chemical plant wastes.
Chromium Preparation Products And Raw materials
Raw materials
Preparation Products
| Supplier | Tel | Country | ProdList | Advantage | Inquiry |
|---|---|---|---|---|---|
| ADVENT CHEMBIO PRIVATE LIMITED | +91-9821524116 +91-9821524116 | Mumbai, India | 353 | 58 | Inquiry |
| Chaitanya Group of Industries | +91-9921920605 +91-9823563605 | Maharashtra, India | 18 | 58 | Inquiry |
| Kamman Group | +91-9619902204 +91-9619902204 | Mumbai, India | 33 | 58 | Inquiry |
| Metsil Exports Ltd | +91-9836009945 +91-9836009945 | West Bengal, India | 13 | 58 | Inquiry |
| ARRAKIS INDUSTRIES LLP | +91 74995 32711 | Maharashtra, India | 1353 | 58 | Inquiry |
| Scientific OEM | +91-22- 2343 7546 / 2341 3094 | New Delhi, India | 1994 | 38 | Inquiry |
| ALPHA CHEMIKA | +91-22-22061123 +91-22-66382501 | Mumbai, India | 1679 | 43 | Inquiry |
| CLEARSYNTH LABS LTD. | +91-22-45045900 | Hyderabad, India | 6257 | 58 | Inquiry |
| Otto Chemie Pvt. Ltd. | +91 9820041841 | Mumbai, India | 5870 | 58 | Inquiry |
| Sisco Research Laboratories Pvt. Ltd. | +91-22-4268 5800 | Mumbai, India | 4315 | 58 | Inquiry |
| Supplier | Advantage |
|---|---|
| ADVENT CHEMBIO PRIVATE LIMITED | 58 |
| Chaitanya Group of Industries | 58 |
| Kamman Group | 58 |
| Metsil Exports Ltd | 58 |
| ARRAKIS INDUSTRIES LLP | 58 |
| Scientific OEM | 38 |
| ALPHA CHEMIKA | 43 |
| CLEARSYNTH LABS LTD. | 58 |
| Otto Chemie Pvt. Ltd. | 58 |
| Sisco Research Laboratories Pvt. Ltd. | 58 |
Related articles
- Discovery and Application of Chromium
- Chromium is a steely gray, lustrous, hard and brittle transition metal highly valued for its corrosion resistance and polished....
- May 27,2024
- Toxicity of Chromium
- Chromium as a metallic element was first discovered over 200 years ago, in 1797. But the history of chromium really began seve....
- Jan 10,2022
- Chromium——Sources of Exposure & Risk Assessments
- Chromium occurs in several different oxidation states from chromium metal to hexavalent chromium (Cr(VI)) compounds. In the en....
- Mar 6,2020
7440-47-3(Chromium)Related Search:
1of4
chevron_right




