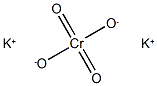
Potassium chromate
- CAS No.
- 7789-00-6
- Chemical Name:
- Potassium chromate
- Synonyms
- CHROMATE;Chromium(VI);KrCrO4;BETZ 0213;tarapacaite;Chromate, CrO;chromateofpotash;chromateofpotass;CHROMATE STANDARD;potassiumchromate6
- CBNumber:
- CB6398284
- Molecular Formula:
- CrK2O4
- Molecular Weight:
- 194.1903
- MOL File:
- 7789-00-6.mol
- MSDS File:
- SDS
- Modify Date:
- 2025/1/27 9:38:02
| Melting point | 971 °C (lit.) |
|---|---|
| Density | 1.00 g/mL at 20 °C |
| bulk density | 1400kg/m3 |
| vapor density | 6.7 (vs air) |
| storage temp. | Store below +30°C. |
| solubility | H2O: soluble |
| form | Solid |
| Specific Gravity | 2.732 |
| color | Yellow |
| PH | 9.0-9.8 (50g/l, H2O, 20℃) |
| Water Solubility | 640 g/L (20 ºC) |
| Merck | 14,7622 |
| Exposure limits |
ACGIH: TWA 0.0002 mg/m3; STEL 0.0005 mg/m3 (Skin) OSHA: Ceiling 0.1 mg/m3 NIOSH: IDLH 15 mg/m3; TWA 0.0002 mg/m3 |
| Stability | Stable. Strong oxidizing agent - contact with combustible materials may lead to fire or violent reaction. Incompatible with strong reducing agents, combustible materials. |
| InChIKey | XMXNVYPJWBTAHN-UHFFFAOYSA-N |
| CAS DataBase Reference | 7789-00-6(CAS DataBase Reference) |
| EPA Substance Registry System | Potassium chromate(VI) (7789-00-6) |
SAFETY
Risk and Safety Statements
| Symbol(GHS) |    GHS07,GHS08,GHS09 |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Signal word | Danger | |||||||||
| Hazard statements | H315-H317-H319-H335-H340-H350i-H410 | |||||||||
| Precautionary statements | P202-P273-P280-P302+P352-P305+P351+P338-P308+P313 | |||||||||
| Hazard Codes | T,N,O | |||||||||
| Risk Statements | 49-46-43-51/53-8-50/53-36/37/38-22-45-52/53-25-42/43-20 | |||||||||
| Safety Statements | 53-45-60-61-36/37-26-23 | |||||||||
| RIDADR | UN 3288 6.1/PG 3 | |||||||||
| WGK Germany | 3 | |||||||||
| RTECS | GB2940000 | |||||||||
| TSCA | Yes | |||||||||
| HazardClass | 5.1 | |||||||||
| PackingGroup | III | |||||||||
| HS Code | 28415000 | |||||||||
| Hazardous Substances Data | 7789-00-6(Hazardous Substances Data) | |||||||||
| NFPA 704 |
|
Potassium chromate price More Price(19)
| Manufacturer | Product number | Product description | CAS number | Packaging | Price | Updated | Buy |
|---|---|---|---|---|---|---|---|
| Sigma-Aldrich(India) | 216615 | Potassium chromate ACS reagent, ≥99.0% | 7789-00-6 | 500G | ₹4189.28 | 2022-06-14 | Buy |
| Sigma-Aldrich(India) | 529508 | Potassium chromate ≥99.95% trace metals basis | 7789-00-6 | 100G | ₹41687.08 | 2022-06-14 | Buy |
| Sigma-Aldrich(India) | 12249 | Potassium chromate puriss., ≥99% | 7789-00-6 | 100G | ₹4459.9 | 2022-06-14 | Buy |
| Sigma-Aldrich(India) | 216615 | Potassium chromate ACS reagent, ≥99.0% | 7789-00-6 | 100G | ₹7523.38 | 2022-06-14 | Buy |
| Sigma-Aldrich(India) | 12249 | Potassium chromate puriss., ≥99% | 7789-00-6 | 500G | ₹7631.63 | 2022-06-14 | Buy |
Potassium chromate Chemical Properties,Uses,Production
Chemical Properties
lemon-yellow crystals
Uses
Potassium chromate (K2CrO4) is soluble in water and is used to make bright yellow inks and paint pigments. It is also used as a reagent in chemical laboratories and as a mordant to “fix” dyes in colored textiles.
Definition
ChEBI: A potassium salt consisting of potassium and chromate ions in a 2:1 ratio.
General Description
Potassium chromate is a yellow crystalline solid. Potassium chromate is soluble in water. The primary hazard is the threat to the environment. Immediate steps should be taken to limit its spread to the environment. Potassium chromate is used in chemical analysis, in making pigments for paints and inks, as a fungicide, and to make other chromium compounds.
Air & Water Reactions
Soluble in water.
Reactivity Profile
Oxidizing agents, such as Potassium chromate , can react with reducing agents to generate heat and products that may be gaseous (causing pressurization of closed containers). The products may themselves be capable of further reactions (such as combustion in the air). The chemical reduction of materials in this group can be rapid or even explosive, but often requires initiation (heat, spark, catalyst, addition of a solvent). Explosive mixtures of inorganic oxidizing agents with reducing agents often persist unchanged for long periods if initiation is prevented. Such systems are typically mixtures of solids, but may involve any combination of physical states. Some inorganic oxidizing agents are salts of metals that are soluble in water; dissolution dilutes but does not nullify the oxidizing power of such materials. Organic compounds, in general, have some reducing power and can in principle react with compounds in this class. Actual reactivity varies greatly with the identity of the organic compound. Inorganic oxidizing agents can react violently with active metals, cyanides, esters, and thiocyanates.
Hazard
Toxic by ingestion and inhalation.
Health Hazard
Inhalation causes local irritation of mucous membranes; continuing nose irritation can result in perforation of nasal septum. Ingestion may cause violent gastroenteritis, circulatory collapse, vertigo, coma, and toxic nephritis; ingestion of excessive quantities can be fatal. Contact with eyes causes severe irritation and conjunctivitis. Repeated or prolonged exposure to dust, mist, or solutions may cause dermatitis; contact with breaks in the skin may cause ``chrome sores'' appearing as slow-healing, hard-rimmed ulcers which leave the area vulnerable to infection.
Fire Hazard
Behavior in Fire: May increase intensity of fire if in contact with combustible materials. Cool containers and spilled material with plenty of water.
Safety Profile
Confirmed carcinogen with experimental tumorigenic data. Poison by ingestion, intravenous, subcutaneous, and intramuscular routes. An experimental teratogen. Other experimental reproductive effects. Human mutation data reported. A powerful oxidizer. When heated to decomposition it emits toxic fumes of K2O. Used as a mordant for wool, in the oxidizing and treatment of dyes on materials. See also CHROMIUM COMPOUNDS.
Potential Exposure
Potassium chromate is used in printing: photomechanical processing; chrome-pigment production; and wool preservative methods; to make dyes, pigments, inks and enamels; as an oxidizing agent; analytical reagent; in electroplating; explosives.
Shipping
UN1479 Oxidizing solid, n.o.s., Hazard Class: 5.1; Labels: 5.1-Oxidizer, Technical Name Required. UN3288 Toxic solids, inorganic, n.o.s., Hazard Class: 6.1; Labels: 6.1-Poisonous materials, Technical Name Required
Purification Methods
Crystallise it from conductivity water (0.6g/mL at 20o), and dry it between 135o and 170o.
Incompatibilities
A powerful oxidizer. Violent reactions with combustibles, organics, powdered metals; or easily oxidizable substances. Contact with hydroxylamine, hydrazine causes explosion.
Potassium chromate Preparation Products And Raw materials
Raw materials
Preparation Products
| Supplier | Tel | Country | ProdList | Advantage | Inquiry |
|---|---|---|---|---|---|
| Nithyasri Chemicals (APURVA CHEMICALS) | +91-2512692130 +91-9323652199 | Maharashtra, India | 75 | 58 | Inquiry |
| RYZE CHEMIE | +91-9702966440 +91-9702966440 | Maharashtra, India | 335 | 58 | Inquiry |
| ARRAKIS INDUSTRIES LLP | +91 74995 32711 | Maharashtra, India | 1353 | 58 | Inquiry |
| JSK Chemicals | +919879767970 | Gujarat, India | 3756 | 58 | Inquiry |
| CLEARSYNTH LABS LTD. | +91-22-45045900 | Hyderabad, India | 6257 | 58 | Inquiry |
| A.J Chemicals | 91-9810153283 | New Delhi, India | 6100 | 58 | Inquiry |
| FINAR LTD | +91 - 2717 - 616717 | New Delhi, India | 660 | 58 | Inquiry |
| Otto Chemie Pvt. Ltd. | +91 9820041841 | Mumbai, India | 5870 | 58 | Inquiry |
| Alfa Aesar | 1 800 209 7001 | Maharashtra, India | 6905 | 58 | Inquiry |
| Sisco Research Laboratories Pvt. Ltd. | +91-22-4268 5800 | Mumbai, India | 4315 | 58 | Inquiry |
| Supplier | Advantage |
|---|---|
| Nithyasri Chemicals (APURVA CHEMICALS) | 58 |
| RYZE CHEMIE | 58 |
| ARRAKIS INDUSTRIES LLP | 58 |
| JSK Chemicals | 58 |
| CLEARSYNTH LABS LTD. | 58 |
| A.J Chemicals | 58 |
| FINAR LTD | 58 |
| Otto Chemie Pvt. Ltd. | 58 |
| Alfa Aesar | 58 |
| Sisco Research Laboratories Pvt. Ltd. | 58 |
7789-00-6(Potassium chromate )Related Search:
1of4
chevron_right




