Chromic acid
- CAS No.
- 7738-94-5
- Chemical Name:
- Chromic acid
- Synonyms
- Cromic acid;CHROMIC ACID;Chromic acid USP/EP/BP;TIANFU-CHEM Chromic acid;dihydroxy(diketo)chromium
- CBNumber:
- CB52130391
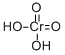
- Molecular Formula:
- CrH2O4
- Molecular Weight:
- 118.01
- MOL File:
- 7738-94-5.mol
- Modify Date:
- 2024/12/18 14:08:52
| Melting point | 196°C |
|---|---|
| Boiling point | 250 °C (decomposes) |
| Density | 2.290 |
| solubility | Methanol (Slightly) |
| form | Liquid |
| color | Clear, orange |
| PH | 3.03(1 mM solution);2.33(10 mM solution);2.06(100 mM solution) |
| Odor | Odorless |
| Water Solubility | HIGHLY Soluble |
| EPA Substance Registry System | Chromic(VI) acid (7738-94-5) |
SAFETY
Risk and Safety Statements
| Symbol(GHS) |    GHS08,GHS07,GHS09 |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Signal word | Danger | |||||||||
| Hazard statements | H400-H317-H350-H410 | |||||||||
| Precautionary statements | P261-P272-P280-P302+P352-P333+P313-P321-P363-P501-P273-P391-P501-P273-P391-P501 | |||||||||
| RIDADR | UN 1463/1755 | |||||||||
| OEB | E | |||||||||
| OEL | TWA: 0.0002 mg/m3 | |||||||||
| Hazardous Substances Data | 7738-94-5(Hazardous Substances Data) | |||||||||
| NFPA 704 |
|
Chromic acid Chemical Properties,Uses,Production
Description
Chromic acid, CrO3, is composed of dark, purplish-red, odorless crystals that are soluble in water. The specific gravity is 2.7, which is heavier than water. It is a powerful oxidizing agent and may explode on contact with organic materials. Chromic acid is a poison, corrosive to the skin, and has a TLV of 0.05 mg/m3 of air. Chromic acid is a known human carcinogen. The four-digit UN identification number is 1463. The NFPA 704 designation is health 3, flammability 0, and reactivity 1. The white section at the bottom of the 704 diamond has an “oxy” prefix, indicating that it is an oxidizer.
Chemical Properties
Chromic acid is a dark purplish-red odorless flakes or crystalline powder
Uses
Chemicals (chromates, oxidizing agents, catalysts), chromium-plating intermediate, medicine (caustic), process engraving, anodizing, ceramic glazes, colored glass, metal cleaning, inks, tanning, paints, textile mordant, etchant for plastics.
Definition
The name is in common use, although the true chromic acid, H2CrO4, exists only in solution.
General Description
Chromic acid is a dark purplish red solid, exists only in solution. The hydrate of chromiumoxide, it is used in electroplating baths. Chromic acid is soluble in water with the release of heat. The material itself is noncombustible but Chromic acid will accelerate the burning of combustible materials. Its solution is corrosive to metals and tissue.
Air & Water Reactions
Water soluble.
Reactivity Profile
A very powerful oxidizing agent, confirmed human carcinogen. Upon contact with reducing reagents Chromic acid can cause a violent explosion, in contact with organic matter Chromic acid may cause a violent oxidation leading to ignition. Dangerously reactive with acetone, alcohols, alkali metals (sodium, potassium), ammonia, arsenic, dimethylformamide, hydrogen sulfide, phosphorus, peroxyformic acid, pyridine, selenium, sulfur, and many other chemicals [Sax, 9th ed., 1996, p. 852]. When mixed with sulfuric acid for glass cleaning operations, used solution in closed bottle may explode due to internal pressure of carbon dioxide arising from contamination by carbon compounds [Bryson, W. R., Chem. Brit., 1975, 11, p. 377].
Hazard
A human carcinogen. A poison. Corrosive to skin. Powerful oxidizing agent, may explode on contact with reducing agents, may ignite on contact with organic materials. Upper respiratory tract irritant.
Health Hazard
Very irritating to eyes and respiratory tract. Ingestion causes severe gastrointestinal symptoms. Contact with eyes or skin causes burns; prolonged contact produces dermatitis (``chrome sores'').
Fire Hazard
Behavior in Fire: Containers may explode
Safety Profile
Confirmed human carcinogen. Poison by subcutaneous route. Mutation data reported. A powerful oxidzer. A powerful irritant of skin, eyes, and mucous membranes. Can cause a dermatitis, bronchoasthma, “chrome holes,” damage to the eyes. Dangerously reactive. Incompatible with acetic acid, acetic anhydride, tetrahydronaphthalene, acetone, alcohols, alkali metals, ammonia, arsenic, bromine penta fluoride, butyric acid, n,ndimethylformamide, hydrogen sulfide, peroxyformic acid, phosphorus, potassium hexacyanoferrate, pyridme, selenium
Potential Exposure
n chromium plating; medicine, ceramic glazers, and paints.
Shipping
UN1463 Chromium trioxide, anhydrous, Hazard Class: 5.1; Labels: 5.1-Oxidizer, 6.1-Poisonous materials, 8-Corrosive material. UN1755 (solution) Chromic acid, solid, Hazard class: 8; Labels: 8-Corrosive material.
Incompatibilities
A strong oxidizer. Aqueous solution is strongly acidic. Reacts with acetic acid, acetic anhydride, acetone, anthracene, chromous sulfide; diethyl ether; dimethyl formamide; ethanol, hydrogen sulfide; methanol, naphthalene, camphor, glycerol, potassium ferricyanide, pyridine, turpentine, combustibles; organics, and other easily oxidized materials (such as paper, wood, sulfur, aluminum, and plastics). Attacks metals in presence of moisture
Waste Disposal
Chemical reduction to chromium(III) can be followed by land fill disposal of the sludge.
Chromic acid Preparation Products And Raw materials
Raw materials
Preparation Products
1of3
chevron_right| Supplier | Tel | Country | ProdList | Advantage | Inquiry |
|---|---|---|---|---|---|
| JAGGO OVERSEAS | +91-9999536088 +91-9999536089 | New Delhi, India | 6 | 58 | Inquiry |
| oswal chemicals | +91-9173644055 +91-9173644055 | Gujarat, India | 66 | 58 | Inquiry |
| VITRAG CHEMICALS PVT LTD | +91-7490053194 +91-7490053194 | Gujarat, India | 47 | 58 | Inquiry |
| Silverline Chemicals | +91-9810339289 +91-9810339289 | Haryana, India | 71 | 58 | Inquiry |
| Vishnu Chemicals Ltd | +91-4023327729 +91-4023327729 | Telangana, India | 4 | 58 | Inquiry |
| Triveni chemicals | 08048762458 | New Delhi, India | 6088 | 58 | Inquiry |
| Quality Deals India | 07942564432 | Hyderabad, India | 8 | 58 | Inquiry |
| Sunayana Chemicals | 08046063253 | Gujarat, India | 24 | 58 | Inquiry |
| Prime Laboratories | 08046047249 | Hyderabad, India | 24 | 58 | Inquiry |
| Kakar Trading Company | 07942553575 | Delhi, India | 9 | 58 | Inquiry |
| Supplier | Advantage |
|---|---|
| JAGGO OVERSEAS | 58 |
| oswal chemicals | 58 |
| VITRAG CHEMICALS PVT LTD | 58 |
| Silverline Chemicals | 58 |
| Vishnu Chemicals Ltd | 58 |
| Triveni chemicals | 58 |
| Quality Deals India | 58 |
| Sunayana Chemicals | 58 |
| Prime Laboratories | 58 |
| Kakar Trading Company | 58 |
7738-94-5(Chromic acid)Related Search:
1of4
chevron_right




