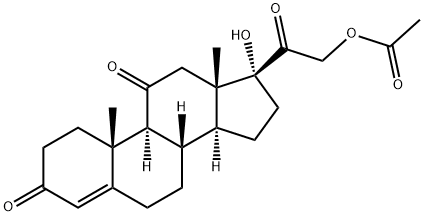
Cortisone acetate
- CAS No.
- 50-04-4
- Chemical Name:
- Cortisone acetate
- Synonyms
- artriona;cortelan;cortisyl;ricortex;NSC 49420;cortistab;biocortacetate;cortoneacetate;irisoneacetate;cortogenacetate
- CBNumber:
- CB9277612
- Molecular Formula:
- C23H30O6
- Molecular Weight:
- 402.48
- MOL File:
- 50-04-4.mol
- MSDS File:
- SDS
- Modify Date:
- 2024/7/2 8:55:41
| Melting point | 237-240 °C(lit.) |
|---|---|
| Boiling point | 577.3±50.0 °C(Predicted) |
| alpha | D25 +164° (c = 0.5 in acetone); D25 +208 to +217° (dioxane) |
| Density | 1.26±0.1 g/cm3(Predicted) |
| refractive index | 212 ° (C=1, MeOH) |
| storage temp. | 2-8°C |
| solubility | Practically insoluble in water, freely soluble in methylene chloride, soluble in dioxan, sparingly soluble in acetone, slightly soluble in ethanol (96 per cent) and in methanol. |
| form | Solid |
| pka | 12.32±0.60(Predicted) |
| color | White to Off-White |
| Water Solubility | 19mg/L(25 ºC) |
| Merck | 14,2539 |
| BRN | 2067543 |
| Stability | Stable. Incompatible with strong oxidizing agents. |
| CAS DataBase Reference | 50-04-4(CAS DataBase Reference) |
| EPA Substance Registry System | Cortisone acetate (50-04-4) |
SAFETY
Risk and Safety Statements
| Symbol(GHS) |  GHS08 |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Signal word | Warning | |||||||||
| Hazard statements | H373-H361 | |||||||||
| Precautionary statements | P260-P314-P501-P201-P202-P281-P308+P313-P405-P501 | |||||||||
| Safety Statements | 22-36/37-24/25 | |||||||||
| WGK Germany | 3 | |||||||||
| RTECS | GM9140000 | |||||||||
| HS Code | 32041200 | |||||||||
| NFPA 704 |
|
Cortisone acetate price More Price(5)
| Manufacturer | Product number | Product description | CAS number | Packaging | Price | Updated | Buy |
|---|---|---|---|---|---|---|---|
| Sigma-Aldrich(India) | C3130 | Cortisone 21-acetate ≥99% | 50-04-4 | 1G | ₹6386.75 | 2022-06-14 | Buy |
| Sigma-Aldrich(India) | C3130 | Cortisone 21-acetate ≥99% | 50-04-4 | 5G | ₹24832.55 | 2022-06-14 | Buy |
| Sigma-Aldrich(India) | C3130 | Cortisone 21-acetate ≥99% | 50-04-4 | 10G | ₹36880.78 | 2022-06-14 | Buy |
| Sigma-Aldrich(India) | PHR1923 | Cortisone Acetate Pharmaceutical Secondary Standard; Certified Reference Material | 50-04-4 | 300MG | ₹20621.63 | 2022-06-14 | Buy |
| Sigma-Aldrich(India) | C3130 | Cortisone 21-acetate ≥99% | 50-04-4 | 25G | ₹74270.33 | 2022-06-14 | Buy |
Cortisone acetate Chemical Properties,Uses,Production
Description
Cortisone acetate is a synthetic glucocorticoid with anti-inflammatory properties. It decreases the size of Bacillus Calmette-Guérin (BCG) vaccine-induced dermal lesions and tuberculin reactions in rabbits when administered at 2 mg/kg on alternate days over the course of 46 days. It also reduces the number and percentage of activated lesion-infiltrating mononuclear cells and decreases the amount of caseous necrosis and ulceration. Cortisone acetate (2.5 mg/kg per day, s.c.) slows tissue regeneration in a rabbit model of wound healing. It also decreases the number of dexamethasone binding sites on isolated human lymphocytes by 30%. Formulations containing cortisone acetate have been used to relieve inflammation, pruritic manifestations of corticosteroid-responsive dermatoses, and in the treatment of immune and allergic disorders.
Chemical Properties
White needle crystal or crystalline powder (acetone), odorless. Stable in the air. Mp239- 241°C, specific rotation [α]20D+164°(0.5%, acetone), [α]23D+169°(chloroform), [α]25D+208°-+217°(dioxane), The ethanol solution of this product has a maximum absorption at a wavelength of 240nm. Soluble in chloroform (1:4), soluble in acetone (1:75), slightly soluble in ethanol and ether, and hardly soluble in water. This product dissolves in sulfuric acid and turns yellow without fluorescence (different from hydrocortisone acetate).
Uses
Cortisone Acetate (Hydrocortisone Acetate EP Impurity D) is a glucocorticoid. Cortisone Acetate is an antiinflammatory agent. Cortisone Acetate is bioavailable and readily converted to the therapeutically active form, Hydrocotisone.
Preparation
Cortisone acetate is prepared from dehydropregnenolone by epoxidation, Wolff's oxidation, mycoxidation, chromic acid oxidation, hydrogen bromide ring-opening, Raney's nickel-catalyzed debromination, iodination, and acetyloxy substitution.
General Description
Cortisone acetate, 21-(acetyloxy)-17-hydroxypregn-4-ene-3,11,20-trione, is the 21-acetate of naturally occurring cortisone with good systemicanti-inflammatory activity and low-to-moderate salt-retentionactivity after its in vivo conversion to hydrocortisoneacetate. This conversion is mediated by 11β-hydroxysteroiddehydrogenase. It is used for the entire spectrum of uses discussedpreviously under the heading, “Therapeutic Uses ofAdrenal Cortex Hormones”—collagen diseases, Addisondisease, severe shock, allergic conditions, chronic lymphocyticleukemia, and many other indications. Cortisone acetateis relatively ineffective topically, mainly because itmust be reduced in vivo to hydrocortisone. Its plasma halflifeis only about 30 minutes, compared with 90 minutes to3 hours for hydrocortisone.
Purification Methods
Crystallise -1cortisone-21-acetate from acetone or CHCl3. The UV has 15,800 M-1cm at 238nm in dioxane. [Sarett J Biol Chem 162 601 1946, Beilstein 8 III 4058, 5 IV 3481.]
References
1. Cortisone acetate in skin disease; local effect in the skin from topical application and local injection. DOI:10.1001/ARCHDERM.1952.01530210056007
2. McCue, R.E., Dannenberg, A.M., Jr., Huguchi, S., et al. The effect of cortisone on the accumulation, activation, and necrosis of macrophages in tuberculous lesions. Inflammation 3(2), 159-176 (1978). DOI:10.1007/BF00910737
3. Joseph, J., and Tydd, M. The effects of cortisone acetate on tissue regeneration in the rabbit’s ear. J. Anat. 115(Pt. 3), 445-460 (1973).
4. Schlechte, J.A., Ginsberg, B.H., and Sherman, B.M. Regulation of the glucocorticoid receptor in human lymphocytes. J. Steroid Biochem. 16(1), 69-74 (1982). DOI:10.1016/0022-4731(82)90145-5
5. Sittig's Pharmaceutical Manufacturing Encyclopedia
6. Textbook of organic medicinal and pharmaceutical chemistry. DOI:10.1002/jps.3030451120
7. Purification of Laboratory Chemicals DOI:10.5860/choice.50-6768
Cortisone acetate Preparation Products And Raw materials
Raw materials
1of4
chevron_rightPreparation Products
| Supplier | Tel | Country | ProdList | Advantage | Inquiry |
|---|---|---|---|---|---|
| CHEMSWORTH | +91-261-2397244 | New Delhi, India | 6707 | 30 | Inquiry |
| TCI Chemicals (India) Pvt. Ltd. | 1800 425 7889 | New Delhi, India | 6778 | 58 | Inquiry |
| CLEARSYNTH LABS LTD. | +91-22-45045900 | Hyderabad, India | 6351 | 58 | Inquiry |
| SynZeal Research Pvt Ltd | +1 226-802-2078 | Gujarat, India | 6522 | 58 | Inquiry |
| HiMedia Laboratories | 91-22-61471919 | Maharashtra, India | 1843 | 58 | Inquiry |
| Pharma Affiliates | 172-5066494 | Haryana, India | 6761 | 58 | Inquiry |
| Opulent Pharma | 08068441147Ext 981 | Gujarat, India | 810 | 58 | Inquiry |
| Pharmaffiliates Analytics & Synthetics (P) Ltd. | 91-172-5066494 | Haryana, India | 631 | 58 | Inquiry |
| Pharmaffiliates Analytics and Synthetics P. Ltd | +91-172-5066494 | Haryana, India | 6773 | 58 | Inquiry |
| Hebei Kangcang new material Technology Co., LTD | +8615713292910 | China | 341 | 58 | Inquiry |
50-04-4(Cortisone acetate)Related Search:
1of4
chevron_right




