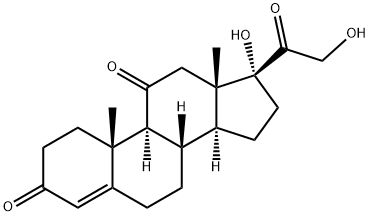
CORTISONE
- CAS No.
- 53-06-5
- Chemical Name:
- CORTISONE
- Synonyms
- KE;Corlin;CORTONE;Cortison;17A 21-DIHYDROXY-4-PREGNEN-3 17 20-TRIONE;Corton;Adreson;Andreson;Cortisal;Cortogen
- CBNumber:
- CB6413305
- Molecular Formula:
- C21H28O5
- Molecular Weight:
- 360.44
- MOL File:
- 53-06-5.mol
- MSDS File:
- SDS
- Modify Date:
- 2023/9/25 13:50:22
| Melting point | 223-228 °C (dec.)(lit.) |
|---|---|
| alpha | D25 +209° (c = 1.2 in 95% alcohol); 25546 +269° (c = 0.125 in benzene); 25546 +248° (c = 0.1 to 0.2 in alcohol) |
| Boiling point | 412.46°C (rough estimate) |
| Density | 1.28±0.1 g/cm3 (20 ºC 760 Torr) |
| refractive index | 210 ° (C=1, EtOH) |
| Flash point | 9℃ |
| storage temp. | -20°C Freezer |
| solubility | Chloroform (Slightly, Heated, Sonicated), DMSO (Slightly), Ethanol (Slightly, Heated) |
| form | Solid |
| pka | 12.37±0.60(Predicted) |
| color | White to Pale Beige |
| Water Solubility | 229.9mg/L(25 ºC) |
| Merck | 2539 |
| LogP | 1.470 |
| CAS DataBase Reference | 53-06-5(CAS DataBase Reference) |
SAFETY
Risk and Safety Statements
| Symbol(GHS) |    GHS02,GHS06,GHS08 |
|---|---|
| Signal word | Danger |
| Hazard statements | H225-H301+H311+H331-H370 |
| Precautionary statements | P210-P260-P280-P301+P310-P311 |
| Hazard Codes | F,T,Xn |
| Risk Statements | 11-23/24/25-39/23/24/25-63 |
| Safety Statements | 22-24/25-45-36/37-16-7 |
| RIDADR | UN1230 - class 3 - PG 2 - Methanol, solution |
| WGK Germany | 3 |
| RTECS | GM9020000 |
| F | 18 |
| HS Code | 2937210000 |
CORTISONE price More Price(4)
| Manufacturer | Product number | Product description | CAS number | Packaging | Price | Updated | Buy |
|---|---|---|---|---|---|---|---|
| Sigma-Aldrich(India) | C-130 | Cortisone solution 100?μg/mL in methanol, ampule of 1?mL, certified reference material, Cerilliant? | 53-06-5 | 1ML | ₹8498.7 | 2022-06-14 | Buy |
| TCI Chemicals (India) | C1317 | Cortisone | 53-06-5 | 1G | ₹3100 | 2022-05-26 | Buy |
| ottokemi | C 2520 | Cortisone 98% | 53-06-5 | 250mg | ₹3069 | 2022-05-26 | Buy |
| ottokemi | C 2520 | Cortisone 98% | 53-06-5 | 1gm | ₹8208 | 2022-05-26 | Buy |
CORTISONE Chemical Properties,Uses,Production
Chemical Properties
Off-White Crystalline Powder
Uses
Cortisone is used for inflammatory processes, allergies, and adrenal insufficiency.
Definition
ChEBI: A C21-steroid that is pregn-4-ene substituted by hydroxy groups at positions 17 and 21 and oxo group at positions 3, 11 and 20.
General Description
Cortisone is a corticosteroid produced in the adrenal glands. Cortisone is administered for short term pain relief and to reduce swelling from inflammation. This Certified Spiking Solution? is applicable in LC-MS/MS applications for endocrinology, clinical chemistry and neonatal screening.
Hazard
Damaging side effects, e.g., sodium retention from ingestion.
Pharmacokinetics
Following oral administration, cortisone acetate and hydrocortisone acetate are completely and rapidly deacetylated by first-pass metabolism. Much of the oral cortisone, however, is inactivated by oxidative metabolism before it can be converted to hydrocortisone in the liver. The pharmacokinetics for hydrocortisone acetate is indistinguishable from that of orally administered hydrocortisone. Oral hydrocortisone is completely absorbed, with a bioavailability of greater than 95% and a half-life of 1 to 2 hours (23).
Clinical Use
Cortisone is administered orally or by intramuscular (IM) injection as its 21-acetate (cortisone acetate).Cortisone acetate or hydrocortisone usually is the corticosteroid of choice for replacement therapy in patients with adrenocortical insufficiency, because these drugs have both glucocorticoid and mineralocorticoid properties.
Metabolism
The metabolism of hydrocortisone has been previously described. Cortisone acetate is slowly absorbed from IM injection sites over a period of 24 to 48 hours and is reserved for patients who are unable to take the drug orally. The acetate ester derivative demonstrates increased stability and has a longer duration of action when administered by IM injection. Thus, smaller doses can be used. Similarly, hydrocortisone may be dispensed as its 21-acetate (hydrocortisone acetate), which is superior to cortisone acetate when injected intra-articularly.
Purification Methods
Crystallise cortisone from 95% EtOH or acetone. The UV has 14,000 M-1cm -1 at 237nm (EtOH). [Beilstein 8 IV 3480, Hems J Pharm Pharmacol 5 409 1953, Beilstein 8 IV 3480.]
CORTISONE Preparation Products And Raw materials
Raw materials
1of2
chevron_rightPreparation Products
| Supplier | Tel | Country | ProdList | Advantage | Inquiry |
|---|---|---|---|---|---|
| Otto Chemie Pvt. Ltd. | +91 9820041841 | Mumbai, India | 5873 | 58 | Inquiry |
| TCI Chemicals (India) Pvt. Ltd. | 1800 425 7889 | New Delhi, India | 6778 | 58 | Inquiry |
| Pharmaffiliates Analytics and Synthetics P. Ltd | +91-172-5066494 | Haryana, India | 6773 | 58 | Inquiry |
| CLEARSYNTH LABS LTD. | +91-22-45045900 | Hyderabad, India | 6351 | 58 | Inquiry |
| A.J Chemicals | 91-9810153283 | New Delhi, India | 6124 | 58 | Inquiry |
| Pharma Affiliates | 172-5066494 | Haryana, India | 6761 | 58 | Inquiry |
| SynZeal Research Pvt Ltd | +1 226-802-2078 | Gujarat, India | 6522 | 58 | Inquiry |
| Clearsynth Labs | 91-22-45045900 | Maharashtra, India | 3889 | 58 | Inquiry |
| Anant Pharmaceuticals Pvt. ltd | +91 8550986868 | Maharashtra, India | 736 | 58 | Inquiry |
| Synthink Research Chemicals | 09158051123 | Pune, India | 398 | 58 | Inquiry |
53-06-5(CORTISONE)Related Search:
1of4
chevron_right




