Adrenosterone
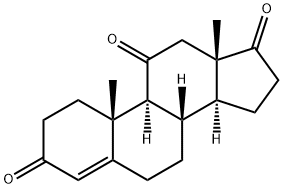
- CAS No.
- 382-45-6
- Chemical Name:
- Adrenosterone
- Synonyms
- 11-KETOANDROSTENEDIONE;Nsc12166;s substance G;ADRENOSTERONE;Andrenosterone;Reichstein'REICHSTEIN'S 'G';(+)-ADRENOSTERONE;Adrenosterone >REICHSTEIN'S ''G''
- CBNumber:
- CB1765508
- Molecular Formula:
- C19H24O3
- Molecular Weight:
- 300.39
- MOL File:
- 382-45-6.mol
- Modify Date:
- 2024/7/2 14:09:54
| Melting point | 219-222 °C(lit.) |
|---|---|
| alpha | 300 º (c=1 in chloroform) |
| Boiling point | 381.62°C (rough estimate) |
| Density | 1.1381 (rough estimate) |
| refractive index | 290 ° (C=0.2, EtOH) |
| storage temp. | Sealed in dry,Room Temperature |
| solubility | Chloroform (Slightly), Ethyl Acetate (Slightly) |
| form | Solid |
| color | White to Off-White |
| optical activity | [α]20/D +300°, c = 1 in chloroform |
| Water Solubility | 98.49mg/L(23.5 ºC) |
| Merck | 14,176 |
| InChIKey | RZRPTBIGEANTGU-IRIMSJTPSA-N |
| CAS DataBase Reference | 382-45-6(CAS DataBase Reference) |
| NIST Chemistry Reference | Androst-4-ene-3,11,17-trione(382-45-6) |
Adrenosterone price More Price(2)
Adrenosterone Chemical Properties,Uses,Production
Chemical Properties
Off-White to Pale Yellow Solid
Uses
Androstenedione is a steroid hormone. It is used to make medicine. Androstenedione is used to increase the production of the hormone testosterone to enhance athletic performance, increase energy, keep red blood cells healthy, enhance recovery and growth from exercise, and increase sexual desire and performance.
benefits
Adrenosterone is sold as a dietary supplement since 2007 as a fat loss and muscle gaining supplement. It is thought to be a competitive selective 11βHSD1 inhibitor, which is responsible for activation of cortisol from cortisone. Thus preventing muscle breakdown, and contributing to a majority of its effects.
Purification Methods
Dissolve adrenosterone i n Me2CO, decolorise it with charcoal, filter, add H2O, Me2CO evaporate and the solid is recrystallised from aqueous EtOH. Also recrystallise it from Et2O or Et2O/pentane and dry it at 110o/0.1mm for 2hours. It can be sublimed under high vacuum. [Reichstein Helv Chim Acta 20 953, 979 1937, Mason et al. J Biol Chem 116 267 1936, Beilstein 7 III 4601.]
Adrenosterone Preparation Products And Raw materials
Raw materials
Preparation Products
| Supplier | Tel | Country | ProdList | Advantage | Inquiry |
|---|---|---|---|---|---|
| A.J Chemicals | 91-9810153283 | New Delhi, India | 6100 | 58 | Inquiry |
| CLEARSYNTH LABS LTD. | +91-22-45045900 | Hyderabad, India | 6257 | 58 | Inquiry |
| TCI Chemicals (India) Pvt. Ltd. | 1800 425 7889 | New Delhi, India | 6768 | 58 | Inquiry |
| Nanjing Shizhou Biotechnology Co., Ltd | +86-15850508050 +86-15850508050 | China | 323 | 58 | Inquiry |
| Shaanxi Dideu Medichem Co. Ltd | +86-029-81138252 +86-18789408387 | China | 3922 | 58 | Inquiry |
| Hebei Mujin Biotechnology Co.,Ltd | +86 13288715578 +8613288715578 | China | 12809 | 58 | Inquiry |
| career henan chemical co | +86-0371-86658258 +8613203830695 | China | 29808 | 58 | Inquiry |
| Hebei Bonster Technology Co.,Limited | +8613315996897 | China | 792 | 58 | Inquiry |
| TargetMol Chemicals Inc. | +1-781-999-5354 +1-00000000000 | United States | 32159 | 58 | Inquiry |
| Hefei TNJ Chemical Industry Co.,Ltd. | +86-0551-65418671 +8618949823763 | China | 34563 | 58 | Inquiry |
382-45-6(Adrenosterone)Related Search:
1of4
chevron_right




