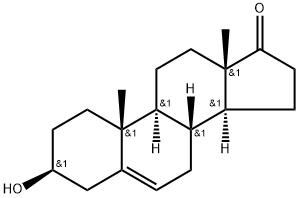
Dehydroepiandrosterone
- CAS No.
- 53-43-0
- Chemical Name:
- Dehydroepiandrosterone
- Synonyms
- DHEA;PRASTERONE;DEHYDROISOANDROSTERONE;DEHYDROEPIANDOSTERONE;TRANS-DEHYDROANDROSTERONE;Dehydroepiandrosterone(DHEA);ANDROSTENOLONE;dehydroepiandrosteron;3β-Hydroxyandrost-5-en-17-one;3BETA-HYDROXYANDROST-5-EN-17-ONE
- CBNumber:
- CB1475670
- Molecular Formula:
- C19H28O2
- Molecular Weight:
- 288.43
- MOL File:
- 53-43-0.mol
- MSDS File:
- SDS
- Modify Date:
- 2025/4/17 16:55:12
| Melting point | 149-151 °C(lit.) |
|---|---|
| alpha | 12 º (c=2, ethanol 96% 25 ºC) |
| Boiling point | 370.65°C (rough estimate) |
| Density | 1.0484 (rough estimate) |
| refractive index | 1.4709 (estimate) |
| Flash point | 9℃ |
| storage temp. | Hormones |
| solubility | insoluble in H2O; ≥13.7 mg/mL in DMSO; ≥58.6 mg/mL in ETOH |
| pka | 15.02±0.60(Predicted) |
| form | Fine Crystalline Powder |
| color | White |
| optical activity | +10.918 (c 1, ethanol) |
| biological source | synthetic (organic) |
| Water Solubility | 21.8mg/L(23.5 ºC) |
| Merck | 2871 |
| BRN | 2058110 |
| InChIKey | FMGSKLZLMKYGDP-USOAJAOKSA-N |
| LogP | 3.230 |
| CAS DataBase Reference | 53-43-0 |
| NIST Chemistry Reference | 5-Androstene-3«beta»-ol-17-one(53-43-0) |
| EPA Substance Registry System | Prasterone (53-43-0) |
SAFETY
Risk and Safety Statements
| Symbol(GHS) |  GHS08 |
|---|---|
| Signal word | Warning |
| Hazard statements | H361fd |
| Precautionary statements | P201-P202-P280-P308+P313-P405-P501 |
| Hazard Codes | Xi,T,F |
| Risk Statements | 36/37/38-39/23/24/25-23/24/25-11 |
| Safety Statements | 26-36-24/25-45-36/37-16-7 |
| RIDADR | UN1230 - class 3 - PG 2 - Methanol, solution |
| WGK Germany | 2 |
| RTECS | BV8396000 |
| HS Code | 29372900 |
Dehydroepiandrosterone price More Price(9)
| Manufacturer | Product number | Product description | CAS number | Packaging | Price | Updated | Buy |
|---|---|---|---|---|---|---|---|
| Sigma-Aldrich(India) | D4000 | trans-Dehydroandrosterone ≥99% | 53-43-0 | 10G | ₹18445.8 | 2022-06-14 | Buy |
| Sigma-Aldrich(India) | D4000 | trans-Dehydroandrosterone ≥99% | 53-43-0 | 25G | ₹25904.23 | 2022-06-14 | Buy |
| Sigma-Aldrich(India) | D4000 | trans-Dehydroandrosterone ≥99% | 53-43-0 | 100G | ₹59494.2 | 2022-06-14 | Buy |
| Sigma-Aldrich(India) | D4000 | trans-Dehydroandrosterone ≥99% | 53-43-0 | 250G | ₹136784.7 | 2022-06-14 | Buy |
| Sigma-Aldrich(India) | 252805 | DHEA - CAS 53-43-0 - Calbiochem Major secretory steroidal product of the adrenal gland. | 53-43-0 | 10G | ₹29650 | 2022-06-14 | Buy |
Dehydroepiandrosterone Chemical Properties,Uses,Production
Description
Dehydroepiandrosterone(DHEA) is an endogenous steroid hormone that is secreted primarily by the adrenal gland and is the most abundant sex steroid. It is a neurohormone; small quantities of DHEA are produced in the brain and declines in serum and CSF with age. (Knopman and Henderson, 2003). Metabolites of DHEA include androstenedione , which subsequently may be metabolized to testosterone or estrone which is an estradiol precursor. In addition to serving as an intermediate in the biosynthesis of sex steroids, DHEA directly modulates a number of cellular and nuclear receptors.
Chemical Properties
white fine crystalline powder. There are two crystal forms. Needle-like crystal, melting point 140-141oC; small leaf-like crystal, melting point 152-153oC. Has right-handedness. Soluble in alcohol, ether, benzene, slightly soluble in chloroform, petroleum ether. In case of digitonin to produce precipitation.
Occurrence
DHEA is naturally occurring in yam (see Wild Yam, p. 596-597).
Uses
Dehydroepiandrosterone (DHEA) is a major C19 steroid produced by the adrenal cortex. It is a major secretory steroidal product of the adrenal gland; secretion progresively declines with aging. May have estrogen-or androgen-like effects depending on the hormonal milieu. Intracellularly converted to androstenedione. It is used in treatment of menopausal syndrome. People living with HIV may be interested in taking DHEA for a variety of reasons, including treatment of depression, increasing bone density, decreasing arterial plaques, improvement of immune function with HIV, increased memory, and increased muscle strength.
Definition
ChEBI: Dehydroepiandrosterone is an androstanoid that is androst-5-ene substituted by a beta-hydroxy group at position 3 and an oxo group at position 17. It is a naturally occurring steroid hormone produced by the adrenal glands. It has a role as an androgen, a human metabolite and a mouse metabolite. It is a 17-oxo steroid, an androstanoid and a 3beta-hydroxy-Delta(5)-steroid.
Manufacturing Process
To a solution of 1 gram of 16-dehydropregnenolon-3β-acetate in 10 ml pyridine is added 0.22 gram of hydroxylamine hydrochloride, and the mixture is allowed to stand at room temperature for four days. One gram of 16dehydropregnenolon-3β-acetate oxime is dissolved in 30 ml of hot dioxane, and then the solution is cooled in an ice bath until about one-half of the dioxane has solidified. Then 1 gram of phosphorus pentachloride is added and the mixture is shaken until all the dioxane has melted. The mixture is maintained at 35°C, for seventy-five minutes, then an excess of ice is added and the solution is again allowed to stand at 35°C. After about thirty minutes, a solution of 5 ml of concentrated hydrochloric acid in 10 ml of water is added, and the mixture is diluted with water, extracted with ether and the ethereal extract washed with dilute sodium hydroxide solution. The ether is removed on a steam bath and the residue is worked up to yield dehydroepiandrosterone.
World Health Organization (WHO)
The World Health Organization has no information further to the above regarding preparations containing prasterone or to indicate that such preparations remain available.
General Description
DHEA is a major secretory steroidal product of the adrenal gland and is a hormonal precursor to both androgens and estrogens. It can also be synthesized using wild yam or soy, but there is no evidence to show that humans are able to increase DHEA levels by consuming these foods. DHEA and its sulfated metabolite, DHEA-S, are negative modulators of GABAA receptors.
Health Hazard
An experimental teratogen.Experimental reproductive effects. Questionablecarcinogen with experimental neoplastigenic data. Whenheated to decomposition it emits acrid smoke andirritating fumes.
Side effects
Side effects may include acne, skin rash, GI upset, hirsuitism, hypertension, and increased HDL. In people with HIV, additional side effects may include fatigue, nasal congestion, and headaches.
Safety
Dehydroepiandrosterone should always be used under the supervision of a medical professional. It is likely safe for people with low DHEA levels to take oral supplements short-term (<6 months) to restore DHEA to normal, but long-term use and doses resulting in high DHEA levels are possibly unsafe. Side effects are often seen with higher doses and longterm use.
Dehydroepiandrosterone Preparation Products And Raw materials
Raw materials
1of2
chevron_rightPreparation Products
1of2
chevron_right| Supplier | Tel | Country | ProdList | Advantage | Inquiry |
|---|---|---|---|---|---|
| Namiex Chemicals Private Limited | +91-1862225256 +91-9988625256 | Punjab, India | 13 | 58 | Inquiry |
| Symbiotec Pharma Lab Pvt Ltd | +91-7316676406 +91-7316676405 | Madhya Pradesh, India | 71 | 58 | Inquiry |
| AKASH PHARMA EXPORTS | +91-9388123451 +91-9846039283 | Kerela, India | 470 | 58 | Inquiry |
| ALPHA CHEMIKA | +91-22-22061123 +91-22-66382501 | Mumbai, India | 1679 | 43 | Inquiry |
| Scientific OEM | +91-22- 2343 7546 / 2341 3094 | New Delhi, India | 1994 | 38 | Inquiry |
| TCI Chemicals (India) Pvt. Ltd. | 1800 425 7889 | New Delhi, India | 6768 | 58 | Inquiry |
| LOBA CHEMIE PVT.LTD. | 91-22-6663 6699 | Mumbai, India | 3075 | 58 | Inquiry |
| Central Drug House(P) Ltd. | 91-11-49404040 | New Delhi, India | 6157 | 58 | Inquiry |
| Pharmaffiliates Analytics and Synthetics P. Ltd | +91-172-5066494 | Haryana, India | 6739 | 58 | Inquiry |
| Spar Chem | 08068970603Ext 837 | Mumbai, India | 26 | 58 | Inquiry |
Related articles
- Dehydroepiandrosterone: A Comprehensive Examination
- Dehydroepiandrosterone (DHEA) and its sulfate (DHEA-S) are the most abundant steroid hormones in the human body.
- Oct 24,2024
- Biological functions and use of Dehydroepiandrosterone (DHEA)
- Dehydroepiandrosterone (DHEA) and its sulfate ester, DHEAS, are the most abundant steroids circulating in human blood.
- Jun 16,2022
- How is dehydroepiandrosterone controlled?
- Dehydroepiandrosterone is an important precursor hormone, and is the most abundant circulating steroid present in the human bo....
- Oct 23,2019
53-43-0(Dehydroepiandrosterone)Related Search:
1of4
chevron_right




