tolciclate
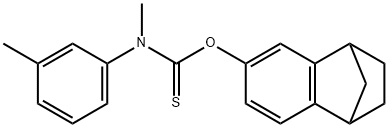
- CAS No.
- 50838-36-3
- Chemical Name:
- tolciclate
- Synonyms
- K 9147;KC 9147;Fungifos;Tolmicen;Kilmicen;Aids029681;Aids-029681;1,4-Methanonaphthalene, carbamothioic acid deriv.;N-Methyl-N-(m-tolyl)thiocarbamic acid O-(1,2,3,4-tetrahydro-1,4-methanonaphthalen-6-yl) ester;N-(3-Methylphenyl)-N-methylthiocarbamic acid O-(1,2,3,4-tetrahydro-1,4-methanonaphthalen-6-yl) ester
- CBNumber:
- CB7893256
- Molecular Formula:
- C20H21NOS
- Molecular Weight:
- 323.45
- MOL File:
- 50838-36-3.mol
- Modify Date:
- 2024/3/16 15:43:42
SAFETY
Risk and Safety Statements
| Toxicity | LD50 in mice, rats, dogs (mg/kg): 4000, 6000, 5000 orally (deCarneri) |
|---|
tolciclate Chemical Properties,Uses,Production
Originator
Tolmicen,Carlo Erba,Italy,1979
Indications
The indications for tolciclate are defined by its narrow spectrum and limited to the treatment of dermatophytoses. Because of inadequate penetration into the nail keratin, it is unlikely to be effective for the treatment of onychomycoses. On average, the efficacy of tolciclate in topical therapy of dermatomycoses is lower than that of most other antimycotics for topical treatment.
Manufacturing Process
Thiophosgene (1.15 g, 0.01 mol) in chloroform (40 ml) was slowly treated at room temperature with sodium 1,4-methano-1,2,3,4-tetrahydro-6- naphthoxide (1.82 g, 0.01 mol). After 30 minutes, N-methyl-m-toluidine (2.42 g, 0.02 mol) in chloroform (40 ml) was added dropwise to the solution so obtained at room temperature. The reaction mixture was stirred for 48 hours at room temperature and then refluxed for 2 hours. The solvent was evaporated, and the residue redissolved in water and extracted repeatedly with diethyl ether. The organic phase was dried (Na2SO4) and evaporated to dryness to give, after crystallization from isopropanol, O-(1,4-methano- 1,2,3,4-tetrahydro-6-naphthyl)-N-methyl-N-(m-tolyl)-thiocarbamate (1.3 g) melting point 92°C to 94°C.
Therapeutic Function
Topical antimycotic
Antimicrobial activity
In vitro, tolciclate has a narrow activity spectrum with good efficacy only towards dermatophytes. Isolates of susceptible species showing primary resistance or developing secondary resistance are rarely observed.
tolciclate Preparation Products And Raw materials
tolciclate Suppliers
| Supplier | Tel | Country | ProdList | Advantage | Inquiry |
|---|---|---|---|---|---|
| TargetMol Chemicals Inc. | +1-781-999-5354 +1-00000000000 | United States | 19892 | 58 | Inquiry |
| Hangzhou MolCore BioPharmatech Co.,Ltd. | +86-057181025280; +8617767106207 | China | 49739 | 58 | Inquiry |
| TargetMol Chemicals Inc. | 4008200310 | China | 24017 | 58 | Inquiry |
| Service Chemical Inc. | 888-895-6920 | Germany | 6373 | 71 | Inquiry |
| CHEMICAL LAND21 | 82- 2 -783 - 8063 | South Korea | 6315 | 74 | Inquiry |
| Supplier | Advantage |
|---|---|
| TargetMol Chemicals Inc. | 58 |
| Hangzhou MolCore BioPharmatech Co.,Ltd. | 58 |
| TargetMol Chemicals Inc. | 58 |
| Service Chemical Inc. | 71 |
| CHEMICAL LAND21 | 74 |





