metixene
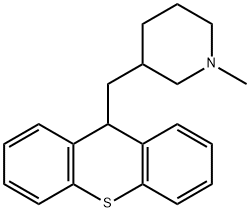
- CAS No.
- 4969-02-2
- Chemical Name:
- metixene
- Synonyms
- Trest;Tremonil;Tremaril;Methixene;Methixart;60 SJ 1977;Aids-001633;115511-20-1 (3S);115511-21-2 (3R);metixene USP/EP/BP
- CBNumber:
- CB7918610
- Molecular Formula:
- C20H23NS
- Molecular Weight:
- 309.47
- MOL File:
- 4969-02-2.mol
- MSDS File:
- SDS
- Modify Date:
- 2023/5/4 17:34:39
| Melting point | <25 °C |
|---|---|
| Boiling point | bp0.07 171-175° |
| Density | 1.118±0.06 g/cm3(Predicted) |
| pka | 9.46±0.10(Predicted) |
| NIST Chemistry Reference | Piperidine, 1-methyl-3-(9h-thioxanthen-9-ylmethyl)-(4969-02-2) |
SAFETY
Risk and Safety Statements
| Symbol(GHS) |   GHS07,GHS09 |
|---|---|
| Signal word | Warning |
| Hazard statements | H302-H410 |
| Precautionary statements | P264-P270-P301+P312-P330-P501-P273-P391-P501 |
| Toxicity | LD50 oral in mouse: 430mg/kg |
metixene Chemical Properties,Uses,Production
Originator
Tremarit Wander W. Germany,Wander ,W. Germany,1960
Manufacturing Process
To 4.9 g of finely pulverized sodium in 50 ml of absolute benzene add dropwise with stirring 12 g of chlorobenzene in 50 ml of absolute benzene. As soon as the exothermic reaction begins, maintain the temperature by cooling between 30° and 35°C, and continue stirring for 2 to 3 hours. To the resulting phenyl sodium add dropwise 19.8 g of thioxanthene in 120 ml of absolute benzene. The slightly exothermic reaction ceases after about 1 to 1? hours.
To this newly formed 9-thioxanthyl sodium add dropwise, with stirring and cooling, 13.1 g of N-methyl-3-chloromethyl-piperidine in 30 to 40 ml of absolute benzene, then continue stirring at about 25°C for 1? hours, and heat subsequently to 40°C for 1 hour. Decompose the resulting mixture by adding carefully a small amount of water, and then extract the newly formed base from the benzene solution by means of dilute hydrochloric acid. The aqueous hydrochloric solution is made alkaline by adding dilute sodium hydroxide, and the thioxanthene base is isolated by extraction with ether. This results in 22 g of a slightly yellow, viscous base of BP 171° to 175°C/0.07 mm.
The base is acidified with alcoholic hydrochloric acid. Alcohol-ether (1:2) is then added and the hydrochloride salt is crystallized as colorless flakes melting at 211° to 213°C.
Therapeutic Function
Spasmolytic
metixene Preparation Products And Raw materials
metixene Suppliers
| Supplier | Tel | Country | ProdList | Advantage | Inquiry |
|---|---|---|---|---|---|
| CONIER CHEM AND PHARMA LIMITED | +8618523575427 | China | 49374 | 58 | Inquiry |
| TargetMol Chemicals Inc. | United States | 38470 | 58 | Inquiry | |
| Amadis Chemical Company Limited | 571-89925085 | China | 131957 | 58 | Inquiry |
| BOC Sciences | 1-631-485-4226; 16314854226 | United States | 14055 | 65 | Inquiry |
| Service Chemical Inc. | 888-895-6920 | Germany | 6350 | 71 | Inquiry |
| Yick-Vic Chemicals & Pharmaceuticals (HK) Ltd | (852)2541 2772 | China | 6206 | 58 | Inquiry |
| Suchem Pharma Co., Ltd. | 86 0577 85505511 13968864715 | China | 79 | 50 | Inquiry |
| American Custom Chemicals Corporation | 858 201 6118 | United States | 6820 | 51 | Inquiry |
| Wonda Science | +1 (514) 581-6866 | United States | 2192 | 50 | Inquiry |
| ABI Chem | United States | 380 | 0 | Inquiry |





