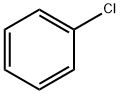
Chlorobenzene
- CAS No.
- 108-90-7
- Chemical Name:
- Chlorobenzene
- Synonyms
- MONOCHLOROBENZENE;Chlorobenzen;Chlorbenzol;Chlorbenzene;CHLOROBENZOL;PHENYL CHLORIDE;cp27;NSC 8433;U.N. 1134;NCI-C54886
- CBNumber:
- CB7249002
- Molecular Formula:
- C6H5Cl
- Molecular Weight:
- 112.56
- MOL File:
- 108-90-7.mol
- MSDS File:
- SDS
- Modify Date:
- 2024/3/14 15:18:27
| Melting point | -45 °C (lit.) |
|---|---|
| Boiling point | 132 °C (lit.) |
| Density | 1.106 g/mL at 25 °C (lit.) |
| vapor density | 3.86 (vs air) |
| vapor pressure | 11.8 mm Hg ( 25 °C) |
| refractive index |
n |
| Flash point | 75 °F |
| storage temp. | 2-8°C |
| solubility | water: soluble0.207 g/L at 20°C |
| form | Liquid |
| color | APHA: ≤30 |
| Odor | Almond like odour |
| Relative polarity | 0.188 |
| explosive limit | 1.3-11%(V) |
| Water Solubility | 0.4 g/L (20 ºC) |
| FreezingPoint | -45.6℃ |
| λmax |
λ: 288 nm Amax: 1.0 λ: 290 nm Amax: 0.40 λ: 300 nm Amax: 0.05 λ: 325 nm Amax: 0.04 λ: 360-400 nm Amax: 0.01 |
| Merck | 14,2121 |
| BRN | 605632 |
| Henry's Law Constant | 1.11, 1.54, 1.81, 2.80, and 3.79 at 2.0, 6.0, 10.0, 18.0, and 25.0 °C, respectively (EPICS-SPME, Dewulf et al., 1999)(x 10-3 atm?m3/mol) |
| Exposure limits | TLV-TWA 75 ppm (~345 mg/m3) (ACGIH, MSHA, OSHA, and NIOSH); IDLH 2400 ppm. |
| Dielectric constant | 5.6(25℃) |
| Stability | Stable. Incompatible with oxidizing agents. Flammable. Attacks some types of plastics, rubber and coatings. |
| LogP | 3 at 20℃ |
| CAS DataBase Reference | 108-90-7(CAS DataBase Reference) |
| NIST Chemistry Reference | Benzene, chloro-(108-90-7) |
| EPA Substance Registry System | Chlorobenzene (108-90-7) |
SAFETY
Risk and Safety Statements
| Symbol(GHS) |    GHS02,GHS07,GHS09 |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Signal word | Warning | |||||||||
| Hazard statements | H226-H315-H332-H411 | |||||||||
| Precautionary statements | P210-P233-P240-P273-P303+P361+P353-P304+P340+P312 | |||||||||
| Hazard Codes | Xn,N,T,F | |||||||||
| Risk Statements | 10-20-51/53-40-39/23/24/25-23/24/25-11 | |||||||||
| Safety Statements | 24/25-61-36/37-45-16-7 | |||||||||
| RIDADR | UN 1134 3/PG 3 | |||||||||
| WGK Germany | 2 | |||||||||
| RTECS | CZ0175000 | |||||||||
| F | 3-10 | |||||||||
| Autoignition Temperature | 590 °C | |||||||||
| TSCA | Yes | |||||||||
| HazardClass | 3 | |||||||||
| PackingGroup | III | |||||||||
| HS Code | 29039190 | |||||||||
| Toxicity | LD50 orally in Rabbit: 2000 - 4000 mg/kg | |||||||||
| IDLA | 1,000 ppm | |||||||||
| NFPA 704 |
|
Chlorobenzene price More Price(47)
| Manufacturer | Product number | Product description | CAS number | Packaging | Price | Updated | Buy |
|---|---|---|---|---|---|---|---|
| Sigma-Aldrich(India) | RTC000099 | Chlorobenzene Pharmaceutical Secondary Standard; Certified Reference Material | 108-90-7 | 20ML | ₹12037.55 | 2022-06-14 | Buy |
| Sigma-Aldrich(India) | PHR1665 | Chlorobenzene Pharmaceutical Secondary Standard; Certified Reference Material | 108-90-7 | 3X1.2ML | ₹8883.1 | 2022-06-14 | Buy |
| Sigma-Aldrich(India) | 8.01791 | Chlorobenzene for synthesis | 108-90-7 | 100ML | ₹5120 | 2022-06-14 | Buy |
| Sigma-Aldrich(India) | 900521 | Chlorobenzene anhydrous, ZerO2?, 99.8% | 108-90-7 | 4X2ML | ₹1920.1 | 2022-06-14 | Buy |
| Sigma-Aldrich(India) | 8.14506 | Chlorobenzene special quality for synthesis | 108-90-7 | 2.5L | ₹14970 | 2022-06-14 | Buy |
Chlorobenzene Chemical Properties,Uses,Production
Chemical Properties
Chlorobenzene, also known as monochlorobenzene or MCB, is a colourless flammable liquid with an aromatic almond-like odor, Chlorination of benzene in the presence of a catalyst (FeCl3 or AICI3) yields chlorobenzene as the first product. It is insoluble in water and miscible with organic solvents. Chlorobenzene has a good solvency for fats, oils, resins, polymers, binders, rubber, and chlorinated rubber. Cellulose ethers dissolve in the presence of small amounts of alcohols; cellulose nitrate is insoluble. Chlorobenzene is a solvent in the production of bitumen and asphalt coatings for building protection.
Physical properties
Clear, colorless, flammable liquid with a sweet almond, medicinal or mothball-like odor. An odor threshold concentration of 210 ppbv was reported by Leonardos et al. (1969). At 40 °C, the lowest concentration at which an odor was detected was 190 μg/L. At 25 °C, the lowest concentration at which a taste was detected was 190 μg/L (Young et al., 1996). The average least detectable odor threshold concentration in water at 60 °C was 0.08 mg/L (Alexander et al., 1982). Cometto-Muiz and Cain (1994) reported an average nasal pungency threshold concentration of 10,553 ppmv. Chlorobenzene can evaporate when exposed to air. It dissolves slightly when mixed with water. It is moderately soluble in water; up to 1,000 milligrams will mix with a liter of water. Chlorobenzene is slightly persistent in water, with a half-life of between 2 to 20 days. It persists in soil (several months), in air (3.5 days), and water (less than 1 day).
Uses
Chlorobenzene is a halogenated benzene that is used as a solvent for paints, as a heat transfer medium, and in the manufacture of phenol, aniline and nitrochlorobenzenes. Now chlorobenzene is mainly used as a solvent for pesticide formulations, diisocyanate manufacture, degreasing automobile parts, and for the production of nitrochlorobenzene and chemical toxicity QSAR research for agricultural pollution.
Definition
ChEBI: Chlorobenzene is the simplest member of the class of monochlorobenzenes, that is benzene in which a single hydrogen has been substituted by a chlorine. It has a role as a solvent.
Preparation
Chlorobenzene is produced by chlorination of benzene in the presence of a catalyst, and is produced as an end product in the reductive chlorination of di- and trichlorobenzenes.
General Description
Chlorobenzene is a colorless to clear, yellowish liquid with a sweet almond-like odor. Flash point 84°F. Practically insoluble in water and somewhat denser than water (9.2 lb / gal). Vapors heavier than air. It can be converted to phenol by reaction with sodium hydroxide under extreme conditions (300°C and 200 atmospheres pressure). Used to make pesticides, dyes, and other chemicals.
Air & Water Reactions
Highly flammable. Insoluble in water.
Reactivity Profile
Chlorobenzene undergoes a sometimes explosive reaction with powdered sodium or phosphorus trichloride + sodium. May react violently with dimethyl sulfoxide. Reacts vigorously with oxidizing agents. Attacks some forms of plastic, rubber and coatings. Forms a shock sensitive solvated salt with silver perchlorate.
Hazard
A possible carcinogen. Avoid inhalation and skin contact. Moderate fire risk. Explosive limits 1.8–9.6%.
Health Hazard
Irritating to skin, eyes and mucous membranes. Repeated exposure of skin may cause dermatitis due to defatting action. Chronic inhalation of vapors or mist may result in damage to lungs, liver, and kidneys. Acute vapor exposures can cause symptoms ranging from coughing to transient anesthesia and central nervous system depression.
Limited information is available on the acute (short-term) effects of chlorobenzene. Acute inhalation exposure of animals to chlorobenzene produced narcosis, restlessness, tremors, and muscle spasms. Chronic (long-term) exposure of humans to chlorobenzene affects the central nervous system (CNS). Signs of neurotoxicity in humans include numbness, cyanosis, hyperesthesia (increased sensation), and muscle spasms. No information is available on the carcinogenic effects of chlorobenzene in humans. EPA has classified chlorobenzene as a Group D, not classifiable as to human carcinogenicity.
Fire Hazard
Flammable liquid; flash point (closed cup) 29°C (84°F); vapor pressure 8.8 torr at 20°C (68°F); autoignition temperature 638°C (1180°F). When heated to decomposition this compound emits toxic fumes of hydrogen chloride gas, CO and CO2.
Chlorobenzene vapors form explosive mixtures with air within the range 1.3-7.1% by volume in air. It is incompatible with strong oxidizing agents and dimethyl sulfoxide. Dimethyl sulfoxide decom poses violently in contact with chloroben zene (NFPA 1997). Many metal perchlorates, such as those of silver and mercury, may form shock-sensitive solvated perchlorates that may explode on impact.
Safety Profile
Suspected carcinogen. Moderately toxic by ingestion and intraperitoneal routes. Experimental teratogenic and reproductive effects. Mutation data reported. Strong narcotic with slight irritant qualities. Dichlorobenzols are strongly narcotic. Little is known of the effects of repeated exposures at lower concentrations, but it may cause hdney and liver damage. The industrial illnesses reported may possibly be due to nitrobenzol. Dangerous fire hazard when exposed to heat or flame. Moderate explosion hazard when exposed to heat or flame. Potentially explosive reaction with powdered sodium or phosphorus trichloride + sodtum. Violent reaction with AgClO4. Reacts vigorously with oxidizers. See also CHLORINATED HYDROCARBONS, AROMATIC. To fight fire, use foam, CO2, dry chemical, water to blanket fire. Associated with EPA Superfund sites
Potential Exposure
Chlorobenzene is used in the manufacture of aniline, phenol, and chloronitrobenzene; as an intermediate in the manufacture of dyestuffs and many pesticides, as a solvent; and emulsifier.
Carcinogenicity
Chlorobenzene was not mutagenic in a variety of bacterial and yeast assays. Existing data suggest that genotoxicity may not be an area of concern for chlorobenzene exposure in humans.
Environmental Fate
Chlorobenzene's production and use as a chemical intermediate, solvent, and heat transfer medium may result in its release to the environment through various waste streams. If released to air, chlorobenzene will exist solely as a vapor in the atmosphere. Photochemically produced hydroxyl radicals will ultimately degrade vapor-phase chlorobenzene in less than 24h.
Biological. In activated sludge, 31.5% of the applied chlorobenzene mineralized to carbon dioxide after 5 d (Freitag et al., 1985). A mixed culture of soil bacteria or a Pseudomonas sp. transformed chlorobenzene to chlorophenol (Ballschiter and Scholz, 1980). Pure microbial cultures isolated from soil hydroxylated chlorobenzene to 2- and 4-chlorophenol (Smith and Rosazza, 1974). Chlorobenzene was statically incubated in the dark at 25 °C with yeast extract and settled domestic wastewater inoculum. At a concentration of 5 mg/L, biodegradation yields at the end of 1 and 2 wk were 89 and 100%, respectively. At a concentration of 10 mg/L, significant degradation with gradual adaptation was observed.Complete degradation was not observed until after the 3rd week of incubation (Tabak et al.,1981).
https://www.epa.gov
Shipping
UN1134 Chlorobenzene, Hazard Class: 3; Labels: 3-Flammable liquid.
Purification Methods
The main impurities are likely to be chlorinated impurities originally present in the *benzene used in the synthesis of chlorobenzene, and also unchlorinated hydrocarbons. A common purification procedure is to wash it several times with conc H2SO4 then with aqueous NaHCO3 or Na2CO3, and water, followed by drying with CaCl2, K2CO3 or CaSO4, then with P2O5, and distilling. It can also be dried with Linde 4A molecular sieve. Passage through, and storage over, activated alumina has been used to obtain low conductance material. [Flaherty & Stern J Am Chem Soc 80 1034 1958, Beilstein 5 H 199, 5 IV 640.]
Incompatibilities
Reacts violently with strong oxidizers; dimethyl sulfoxide; sodium powder; silver perchlorate; causing fire and explosion hazard. Attacks some plastics, rubber, and coatings. Decomposes on heating, producing phosgene and hydrogen chloride fumes.
Waste Disposal
Incineration, preferably after mixing with another combustible fuel; care must be exercised to assure complete combustion to prevent the formation of phosgene; an acid scrubber is necessary to remove the halo acids produced.
Chlorobenzene Preparation Products And Raw materials
Raw materials
1of2
chevron_rightPreparation Products
1of8
chevron_right| Supplier | Tel | Country | ProdList | Advantage | Inquiry |
|---|---|---|---|---|---|
| ANJI BIOSCIENCES | +91-9000100077 +91-9000100077 | Hyderabad, India | 430 | 58 | Inquiry |
| JSK Chemicals | +919879767970 | Gujarat, India | 3756 | 58 | Inquiry |
| CNS CHEMICALS | +91-9381957258 +91-9381957258 | Telangana, India | 354 | 58 | Inquiry |
| Ultra Chemical Works | +91-9820078105 +91-9820078105 | Mumbai, India | 124 | 58 | Inquiry |
| Chemieorganic Chemicals (I) Pvt Ltd | +91-9322792095 +91-9322792095 | Maharashtra, India | 15 | 58 | Inquiry |
| Globus Pharmachem (Para Products Pvt Ltd) | +91-1204198900 +91-9899370547 | Uttar Pradesh, India | 22 | 58 | Inquiry |
| Ridhdhi Sidhdhi Chemicals | +91 9909006960 +91 9909006962 | Gujarat, India | 13 | 58 | Inquiry |
| Chirag Organics Pvt Ltd | +91-9327755066 +91-9820355066 | Mumbai, India | 11 | 58 | Inquiry |
| Aarti Industries Limited (AIL) | +91-84596144931 +91-9920899935 | Maharashtra, India | 235 | 58 | Inquiry |
| Panoli Intermediates (India) Pvt., Limited. (Kutch Chemical Industries Ltd.) | +91-2652397013 +91-7226032610 | Gujarat, India | 45 | 58 | Inquiry |
Related articles
- Uses of Chlorobenzene
- Chlorobenzene is a colorless, flammable liquid with an aromatic, almond-like odor. Some of it will dissolve in water, but it r....
- Jan 6,2022
108-90-7(Chlorobenzene)Related Search:
1of4
chevron_right




