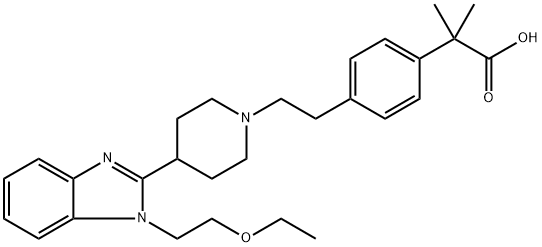
Bilastine
- CAS No.
- 202189-78-4
- Chemical Name:
- Bilastine
- Synonyms
- Bilastine;Bilastin;2-(4-(2-(4-(1-(2-ETHOXYETHYL)-1H-BENZO[D ]IMIDAZOL-2-YL) PIPERIDIN-1-YL)ETHY)PHEN YL)-2-METHYLPROPANOIC ACID;Bilaxten;Bilatex;Bilasten;Bilestin;Bilastene;Bilastine -IP/BP/;Bilastine (Form-I)
- CBNumber:
- CB81011081
- Molecular Formula:
- C28H37N3O3
- Molecular Weight:
- 463.62
- MOL File:
- 202189-78-4.mol
- Modify Date:
- 2025/3/10 11:22:48
| Melting point | 202 °C |
|---|---|
| Boiling point | 639.1±55.0 °C(Predicted) |
| Density | 1.16±0.1 g/cm3(Predicted) |
| storage temp. | Sealed in dry,Room Temperature |
| Water Solubility | Insoluble in water |
| solubility | DMSO:49.3(Max Conc. mg/mL);106.34(Max Conc. mM) |
| pka | 4.40±0.10(Predicted) |
| form | powder to crystal |
| color | White to Light yellow |
SAFETY
Risk and Safety Statements
| Symbol(GHS) |  GHS07 |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Signal word | Warning | |||||||||
| Hazard statements | H302 | |||||||||
| Precautionary statements | P280-P305+P351+P338 | |||||||||
| HS Code | 2933.39.4100 | |||||||||
| NFPA 704 |
|
Bilastine price More Price(2)
Bilastine Chemical Properties,Uses,Production
Description
Bilastine, a potent and selective histamine H1 receptor antagonist, was
approved in Europe in 2010 for the treatment of allergic rhinoconjunctivitis
(AR) and urticaria (hives or skin rash). The original synthesis of bilastine involves alkylation of 2-piperidinyl-1H-benzimidazole with a phenethyltosylate,
the para position of which is substituted with a dimethyloxazoline
moiety serving as a masked carboxylic acid group. Alkylation of the
benzimidazole nitrogen with 2-chloroethyl ethyl ether followed by
unmasking of the oxazoline moiety with sulfuric acid provided bilastine.
In two major clinical trials, bilastine was effective
at relieving allergic rhinitis as assessed by measuring the severity of nasal (obstruction, rhinorrhea, itching, sneezing) and nonnasal (ocular
itching, tearing, ocular redness, itching of ears, and/or palate) symptoms.
Uses
Bilastine is a novel, nonsedating H1-antihistamine developed for symptomatic treatment of allergic rhinitis and chronic idiopathic urticaria.
Clinical Use
Bilastine is a selective histamine H1 antagonist approved for the treatment of allergic rhinoconjunctivitis and urticaria (hives). This drug, which has proven to be well tolerated in toxicology profiling, 46 was discovered by the Spainsh firm FAES Farma and was approved by the European Union in 2010.
Bilastine Preparation Products And Raw materials
Raw materials
Preparation Products
| Supplier | Tel | Country | ProdList | Advantage | Inquiry |
|---|---|---|---|---|---|
| Jigs Chemical ltd | +919099003427 | Gujarat, India | 239 | 58 | Inquiry |
| ALMELO PRIVATE LIMITED | +91-9100991345 +91-8500807769 | Telangana, India | 114 | 58 | Inquiry |
| Anant Pharmaceuticals Pvt Ltd | +91-8550986868 +91-9485998001 | Haryana, India | 460 | 58 | Inquiry |
| Viruj Pharmaceuticals Pvt Ltd | +91-9441591589 +91-9441591589 | Hyderabad, India | 33 | 58 | Inquiry |
| Flax Laboratories | +91-9867665132 +91-9867665132 | Maharashtra, India | 44 | 58 | Inquiry |
| Metrochem API Private Limited | +91-4069069999 +91-4069069999 | Telangana, India | 78 | 58 | Inquiry |
| Meenaakshi Molecules Pvt Ltd | +91-9121311126 +91-9121311126 | Telangana, India | 45 | 58 | Inquiry |
| Sekhment pharmaventures | +91-9030088669 +91-9030088669 | Mumbai, India | 105 | 58 | Inquiry |
| Viyash Life Sciences Pvt Ltd | +91-4023635052 +91-4023635052 | Telangana, India | 213 | 58 | Inquiry |
| Aalidhra Pharmachem Pvt Ltd | +91-9879180894 +91-9879180894 | Gujarat, India | 38 | 58 | Inquiry |
202189-78-4(Bilastine)Related Search:
1of4
chevron_right




