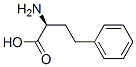
L-Homophe-OH
- CAS No.
- 943-73-7
- Chemical Name:
- L-Homophe-OH
- Synonyms
- HOMOPHENYLALANINE;(S)-2-AMINO-4-PHENYLBUTANOIC ACID;HPHE;LHPA;(S)-APBA;H-HPH-OH;H-HFE-OH;L-HPhe-OH;L-HOMOPHE;H-L-HPA-OH
- CBNumber:
- CB8206268
- Molecular Formula:
- C10H13NO2
- Molecular Weight:
- 179.22
- MOL File:
- 943-73-7.mol
- Modify Date:
- 2024/2/6 18:17:50
| Melting point | >300 °C(lit.) |
|---|---|
| alpha | 45 º (C=1, 3N HCl 19 ºC) |
| Boiling point | 311.75°C (rough estimate) |
| Density | 1.1248 (rough estimate) |
| refractive index | 44 ° (C=1, 3mol/L HCl) |
| storage temp. | Keep in dark place,Inert atmosphere,Room temperature |
| solubility | Soluble in dilute aqueous acid. |
| form | Solid |
| pka | 2.32±0.10(Predicted) |
| color | White to Off-White |
| InChI | InChI=1/C10H13NO2/c11-9(10(12)13)7-6-8-4-2-1-3-5-8/h1-5,9H,6-7,11H2,(H,12,13)/t9-/s3 |
| InChIKey | JTTHKOPSMAVJFE-VIFPVBQESA-N |
| SMILES | C(O)(=O)[C@H](CCC1=CC=CC=C1)N |&1:3,r| |
| CAS DataBase Reference | 943-73-7(CAS DataBase Reference) |
SAFETY
Risk and Safety Statements
| Symbol(GHS) |  GHS07 |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Signal word | Warning | |||||||||
| Hazard statements | H302-H312-H315-H319-H332-H335 | |||||||||
| Precautionary statements | P261-P280-P305+P351+P338 | |||||||||
| Hazard Codes | Xi | |||||||||
| Safety Statements | 22-24/25 | |||||||||
| WGK Germany | 3 | |||||||||
| HS Code | 29224999 | |||||||||
| NFPA 704 |
|
L-Homophe-OH price More Price(6)
| Manufacturer | Product number | Product description | CAS number | Packaging | Price | Updated | Buy |
|---|---|---|---|---|---|---|---|
| TCI Chemicals (India) | H0985 | L-Homophenylalanine | 943-73-7 | 100MG | ₹2000 | 2022-05-26 | Buy |
| TCI Chemicals (India) | H0985 | L-Homophenylalanine | 943-73-7 | 1G | ₹3000 | 2022-05-26 | Buy |
| SRL | 78523 | L-Homophenylalanine extrapure, 97% | 943-73-7 | 500mg | ₹1780 | 2022-05-26 | Buy |
| SRL | 78523 | L-Homophenylalanine extrapure, 97% | 943-73-7 | 1Gms | ₹2970 | 2022-05-26 | Buy |
| SRL | 78523 | L-Homophenylalanine extrapure, 97% | 943-73-7 | 5Gms | ₹7120 | 2022-05-26 | Buy |
L-Homophe-OH Chemical Properties,Uses,Production
Chemical Properties
White Solid
Application
L-homophenylalanine is used almost exclusively as single stereoisomer in pharmaceutical drug production. L-homophenylalanine as a common building block, due to the presence of L-homophenylalanine moiety as the central pharmacophore unit. L-homophenylalanine is also an important chiral intermediate to synthesize a variety of novel pharmaceuticals including β-lactam antibiotics, acetylcholinesterase inhibitor and neutral endopeptidace (NEP) inhibitor which retained their status as major contributors to human health preservation. L-homophenylalanine acts as a precursor for the synthesis of NEP inhibitors, which complements the effects of ACE inhibitors when used simultaneously, potentiates the outcome in the management of hypertension and congestive heart failure[1].
Uses
L-Homophenylalanine is used as a precursor in the pharmaceutical industry for the production of angiotensin-converting enzyme (ACE) and R-(-)-Homophenylalanin-ethylester. It is also involved in the synthesis of NEPA [(S,S)-N-(1-Ethoxycarbonyl-3-phenylpropyl)alanine], which is a key intermediate to prepare the ACE-inhibitors: enalapril, delapril, quinapril and ramipril. It acts as an anti-tumor reagent.
Definition
ChEBI: A non-proteinogenic L-alpha-amino acid that is an analogue of L-phenylalanine having a 2-phenylethyl rather than a benzyl side-chain.
Production Methods
Asymmetric reduction of prochiral ketone remains one of the most investigated methods to date for production of chiral L-homophenylalanine. One of the most established method for synthesizing L-homophenylalanine on a laboratory scale was carried out via enzyme-catalyzed asymmetric synthesis of keto acids. In this method, prochiral ketone was converted via reductive amination to enantiopure products with bulky side chains by addition of biocatalysts such as L-homophenylalanine dehydrogena. in the presence of cofactor. L-phenylalanine dehydrogenase is by and large preferred as it has mainly a catabolic function and accepts a wide variety of keto acids as substrates hence it is generally employed in the synthesis of L-homophenylalanine which carries very bulky side chains[1].
References
[1] Ahmad A, et al. Sustainable biocatalytic synthesis of L-homophenylalanine as pharmaceutical drug precursor. Biotechnology Advances, 2009; 27: 286-296.
L-Homophe-OH Preparation Products And Raw materials
Raw materials
1of2
chevron_rightPreparation Products
| Supplier | Tel | Country | ProdList | Advantage | Inquiry |
|---|---|---|---|---|---|
| JSK Chemicals | +919879767970 | Gujarat, India | 3756 | 58 | Inquiry |
| A.J Chemicals | 91-9810153283 | New Delhi, India | 6124 | 58 | Inquiry |
| Triveni chemicals | 08048762458 | New Delhi, India | 6093 | 58 | Inquiry |
| Anand Agencies | 91-20-24454597 | Maharashtra, India | 2337 | 58 | Inquiry |
| Sisco Research Laboratories Pvt. Ltd. | +91-22-4268 5800 | Mumbai, India | 4317 | 58 | Inquiry |
| TCI Chemicals (India) Pvt. Ltd. | 1800 425 7889 | New Delhi, India | 6778 | 58 | Inquiry |
| Pharmaffiliates Analytics & Synthetics (P) Ltd. | 91-172-5066494 | Haryana, India | 631 | 58 | Inquiry |
| Souvenier Chemicals | 08048372762Ext 645 | Mumbai, India | 396 | 58 | Inquiry |
| Sisco Resech Laboratories Private Limited | +91-22-4268 5800 | Mumbai, India | 655 | 58 | Inquiry |
| DONBOO AMINO ACID COMPANY | +8613063595538 | China | 9365 | 58 | Inquiry |





