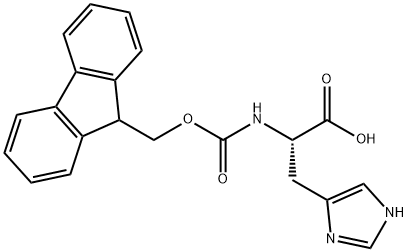
FMOC-HIS-OH
- CAS No.
- 116611-64-4
- Chemical Name:
- FMOC-HIS-OH
- Synonyms
- Fmoc-His;FMOC-HIS-OH;Fmoc-L-His-OH;FMOC-HISTIDINE;FMOC-L-HISTIDINE;Nα-Fmoc-L-histidine;N-a-Fmoc-L-histidine;N-α-Fmoc-L-histidine;FMOC-HIS-OH USP/EP/BP;Nα-Fmoc-L-histidine99%
- CBNumber:
- CB8687841
- Molecular Formula:
- C21H19N3O4
- Molecular Weight:
- 377.39
- MOL File:
- 116611-64-4.mol
- Modify Date:
- 2024/7/3 10:46:41
| Melting point | 171-174 °C(Solv: methanol (67-56-1); water (7732-18-5)) |
|---|---|
| Boiling point | 706.7±60.0 °C(Predicted) |
| Density | 1.372±0.06 g/cm3(Predicted) |
| storage temp. | Sealed in dry,2-8°C |
| solubility | DMSO (Slightly), DMF (Slightly, Sonicated), Methanol (Very Slightly, Heated) |
| pka | 3.26±0.10(Predicted) |
| form | Solid |
| color | White |
| InChIKey | SIRPVCUJLVXZPW-IBGZPJMESA-N |
| SMILES | C(O)(=O)[C@H](CC1N=CNC=1)NC(OCC1C2=C(C=CC=C2)C2=C1C=CC=C2)=O |
SAFETY
Risk and Safety Statements
| Symbol(GHS) |  GHS07 |
|---|---|
| Signal word | Warning |
| Hazard statements | H302-H315-H319-H335 |
| Precautionary statements | P261-P305+P351+P338 |
| HS Code | 29332900 |
FMOC-HIS-OH Chemical Properties,Uses,Production
Description
Nα-Fmoc-L-histidine (FMOC-HIS-OH) holds great significance as a synthetic amino acid extensively employed in peptide synthesis. Its versatility makes it a highly sought-after reagent, widely used in various synthetic processes to create peptides, proteins, and even small molecules. Moreover, it catalyzes peptide and protein synthesis, further enhancing its utility. It is pivotal in unravelling biological processes such as enzymatic catalysis, protein structure, and drug design. Notably, this remarkable amino acid aids in understanding cell signalling pathways, which bear paramount importance in comprehending the mechanisms underlying diseases. The mechanism of action for Nα-Fmoc-L-histidine revolves around its ability to form essential peptide bonds. These bonds arise through a reaction with specific reagents, like DCC, leading to the creation of peptides. Subsequently, deprotection of the peptide bond occurs with the addition of a base, such as sodium hydroxide, followed by an acid, like trifluoroacetic acid.
Uses
Nα-Fmoc-L-histidine is an N-Fmoc-protected form of L-Histidine (H456010). L-Histidine is an essential amino acid that plays an important role in mitochondrial glutamine transport and has potential of abolishing oxidative stress caused by brain edema.L-Histidine promotes zinc uptake in human erythrocyes and also has potential as an antioxidant therapy for acute mammary inflammation in cattle.
FMOC-HIS-OH Preparation Products And Raw materials
| Supplier | Tel | Country | ProdList | Advantage | Inquiry |
|---|---|---|---|---|---|
| A.J Chemicals | 91-9810153283 | New Delhi, India | 6124 | 58 | Inquiry |
| Sisco Research Laboratories Pvt. Ltd. | +91-22-4268 5800 | Mumbai, India | 4317 | 58 | Inquiry |
| Triveni chemicals | 08048762458 | New Delhi, India | 6093 | 58 | Inquiry |
| Sichuan HongRi Pharma-Tech Co.,Ltd | +86-028-64841719 +8617390183901 | China | 1358 | 58 | Inquiry |
| Capot Chemical Co.,Ltd. | 571-85586718 +8613336195806 | China | 29798 | 60 | Inquiry |
| ATK CHEMICAL COMPANY LIMITED | +undefined-21-51877795 | China | 32760 | 60 | Inquiry |
| career henan chemical co | +86-0371-86658258 +8613203830695 | China | 29901 | 58 | Inquiry |
| Chongqing Chemdad Co., Ltd | +86-023-6139-8061 +86-86-13650506873 | China | 39916 | 58 | Inquiry |
| CONIER CHEM AND PHARMA LIMITED | +8618523575427 | China | 49391 | 58 | Inquiry |
| Career Henan Chemica Co | +86-0371-86658258 +8613203830695 | China | 30253 | 58 | Inquiry |
116611-64-4(FMOC-HIS-OH)Related Search:
1of4
chevron_right




