4-Acetamidobenzenesulfonyl azide
- CAS No.
- 2158-14-7
- Chemical Name:
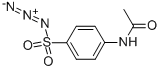
- 4-Acetamidobenzenesulfonyl azide
- Synonyms
- P-ABSA;P-ACETAMIDOBENZENESULFONYL AZIDE;N-(4-azidosulfonylphenyl)acetamide;DKSI122;DKFELEX0337;4-AcetamidobenzenesuL;4-acetamidobenzenesulphon;4-Acetamidobenzenesulfonyl;4-ACETAMIDENZENESULFONYLAZIDE;Acetaminobenzenesulfonyl azide
- CBNumber:
- CB8746801
- Molecular Formula:
- C8H8N4O3S
- Molecular Weight:
- 240.24
- MOL File:
- 2158-14-7.mol
- MSDS File:
- SDS
- Modify Date:
- 2025/1/27 9:38:02
| Melting point | 107-111 °C (lit.) |
|---|---|
| storage temp. | 2-8°C |
| form | powder to crystal |
| color | White to Amber to Dark purple |
| Water Solubility | Insoluble in water. |
| BRN | 2219568 |
| InChI | InChI=1S/C8H8N4O3S/c1-6(13)10-7-2-4-8(5-3-7)16(14,15)12-11-9/h2-5H,1H3,(H,10,13) |
| InChIKey | NTMHWRHEGDRTPD-UHFFFAOYSA-N |
| SMILES | C1(S(=O)(=O)N=[N+]=[N-])=CC=C(NC(=O)C)C=C1 |
| CAS DataBase Reference | 2158-14-7(CAS DataBase Reference) |
SAFETY
Risk and Safety Statements
| Symbol(GHS) |  GHS07 |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Signal word | Warning | |||||||||
| Hazard statements | H315-H319-H335 | |||||||||
| Precautionary statements | P261-P264-P271-P280-P302+P352-P305+P351+P338 | |||||||||
| Hazard Codes | Xi | |||||||||
| Risk Statements | 36/37/38 | |||||||||
| Safety Statements | 26-37/39 | |||||||||
| WGK Germany | 3 | |||||||||
| HS Code | 29350090 | |||||||||
| NFPA 704 |
|
4-Acetamidobenzenesulfonyl azide price More Price(7)
| Manufacturer | Product number | Product description | CAS number | Packaging | Price | Updated | Buy |
|---|---|---|---|---|---|---|---|
| Sigma-Aldrich(India) | 404764 | 4-Acetamidobenzenesulfonyl azide 97% | 2158-14-7 | 5G | ₹1948.5 | 2022-06-14 | Buy |
| Sigma-Aldrich(India) | 404764 | 4-Acetamidobenzenesulfonyl azide 97% | 2158-14-7 | 25G | ₹6440.88 | 2022-06-14 | Buy |
| TCI Chemicals (India) | A1786 | 4-Acetamidobenzenesulfonyl Azide | 2158-14-7 | 5G | ₹2400 | 2022-05-26 | Buy |
| TCI Chemicals (India) | A1786 | 4-Acetamidobenzenesulfonyl Azide | 2158-14-7 | 25G | ₹6800 | 2022-05-26 | Buy |
| TCI Chemicals (India) | A1786 | 4-Acetamidobenzenesulfonyl Azide | 2158-14-7 | 100G | ₹16500 | 2022-05-26 | Buy |
4-Acetamidobenzenesulfonyl azide Chemical Properties,Uses,Production
Uses
4-Acetamidobenzenesulfonyl azide is used as a hydroazidation catalyst for facile preparation of organoazides. It is used as a reagent for synthesis of: monosaccharide-derived alcohols, a late-stage intermolecular C-H olefination, intramolecular isomuenchnone cycloaddition approach to antitumor agents, rhodium-catalyzed carbene cyclization cycloaddition cascade reaction of vinylsulfonates and Suzuki-Miyaura cross coupling reaction.
Synthesis
To a solution of CCA(0.1548 g, 0.6 mmol) in tetrahydrofuran (3-5 mL), PPh3 (0.5246 g, 2 mmol)was added at 0-5°C with stirring. A white suspension was formed to which p-toluenesulfonic acid (0.1720 g, 1 mmol) was added and stirring continued for 15 min. NaN3 (0.065 g, 1 mmol) was added and the temperature was raised up to room temperature. Stirring was continued for 1 min at room temperature. After completion of the reaction (TLC), the reaction mixture was concentrated, washed with EtOAc (4-6 mL), and cold distilled water (5 mL). The organic layer was dried with anhydrous Na2SO4, passed through a short silica-gel column using n-hexane/ethylacetate (10/1) as eluent. 4-Acetamidobenzenesulfonyl azide was obtained after removing the solvent under reduced pressure.
structure and hydrogen bonding
Under ambient conditions, 4-acetamidobenzenesulfonyl azide (4-ABSA) belongs to a monoclinic structure with a P21 space group and cell parameters of a = 8.0529 Å, b = 22.988 Å, c = 8.3123 Å, β=93.534°, respectively. The hydrogen-bonding interactions allow molecules to pair up and form π-stacked dimers. The studies of NH4N3 have reported that the hydrogen bonds are affected by the rotation of azide ions with increasing pressure. The cooperativity of hydrogen bonds and π-stacking interactions allow 4-ABSA to become an attractive candidate for studying the influence of non-covalent interactions within the organic azides in the compression process. In addition, the bent azide groups of 4-ABSA are crucial for electron orbit hybridization and nitrogen polymerization.
References
[1] Junru Jiang. “High-Pressure Studies of 4-Acetamidobenzenesulfonyl Azide: Combined Raman Scattering, IR Absorption, and Synchrotron X-ray Diffraction Measurements.” The Journal of Physical Chemistry B 120 46 (2016): 12015–12022.
4-Acetamidobenzenesulfonyl azide Preparation Products And Raw materials
Raw materials
Preparation Products
| Supplier | Tel | Country | ProdList | Advantage | Inquiry |
|---|---|---|---|---|---|
| GLR Innovations | +91 9891111994 | New Delhi, India | 4535 | 58 | Inquiry |
| REDDY N REDDY PHARMACEUTICALS | +91-9848096759 +91-9848096759 | Hyderabad, India | 1989 | 58 | Inquiry |
| Innovative Labs | +91-9885794886 +91-9885794886 | Telangana, India | 219 | 58 | Inquiry |
| Sakam Finechem Pvt., Ltd. | 91-40-40271222 | Hyderabad, India | 556 | 58 | Inquiry |
| Vihasifinechem Pvt Ltd | -40110230 | Hyderabad, India | 29 | 58 | Inquiry |
| Ms Life Sciences | 08048372386Ext 826 | Hyderabad, India | 341 | 58 | Inquiry |
| Innovative Labs | 91--9885794886 | Mallapur, India | 110 | 58 | Inquiry |
| Vihasibio Sciences Private Limited | -40110230 | Hyderabad, India | 391 | 58 | Inquiry |
| TCI Chemicals (India) Pvt. Ltd. | 1800 425 7889 | New Delhi, India | 6768 | 58 | Inquiry |
| Alfa Aesar | 1 800 209 7001 | Maharashtra, India | 6905 | 58 | Inquiry |
| Supplier | Advantage |
|---|---|
| GLR Innovations | 58 |
| REDDY N REDDY PHARMACEUTICALS | 58 |
| Innovative Labs | 58 |
| Sakam Finechem Pvt., Ltd. | 58 |
| Vihasifinechem Pvt Ltd | 58 |
| Ms Life Sciences | 58 |
| Innovative Labs | 58 |
| Vihasibio Sciences Private Limited | 58 |
| TCI Chemicals (India) Pvt. Ltd. | 58 |
| Alfa Aesar | 58 |
Related articles
- What is 4-Acetamidobenzenesulfonyl azide?
- 4-Acetamidobenzenesulfonyl azide is an important organic intermediate (building block) to synthetize substituted amidobenzene ....
- Feb 18,2020
2158-14-7(4-Acetamidobenzenesulfonyl azide)Related Search:
1of4
chevron_right




