Maytansine
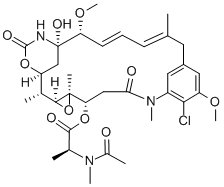
- CAS No.
- 35846-53-8
- Chemical Name:
- Maytansine
- Synonyms
- NSC 153858;Maytansine;maitansine;Maitansina;Microtubule/Tubulin,tumor,mitotic,microtubule-targeted,arrest,Inhibitor,potent,subnanomolar,Maytansine,inhibit;(14S,16S,32S,33S,2R,4S,10E,12E,14R)-86-Chloro-14-hydroxy-85,14-dimethoxy-33,2,7,10-tetramethyl-12,6-dioxo-7-aza-1(6,4)-oxazinana-3(2,3)-oxirana-8(1,3)-benzenacyclotetradecaphane-10,12-dien-4-yl N-acetyl-N-methyl-L-alaninate
- CBNumber:
- CB8903295
- Molecular Formula:
- C34H46ClN3O10
- Molecular Weight:
- 692.2
- MOL File:
- 35846-53-8.mol
- MSDS File:
- SDS
- Modify Date:
- 2024/4/19 13:39:53
| Melting point | 183.5-184℃ |
|---|---|
| alpha | D26 -145° (c = 0.055 in chloroform) |
| Boiling point | 895.1±65.0 °C(Predicted) |
| Density | 1.1049 (rough estimate) |
| refractive index | 1.6000 (estimate) |
| storage temp. | Store at -20°C |
| solubility | DMF: 1 mg/ml; DMSO: 10 mg/ml; Ethanol: 2 mg/ml; PBS (pH 7.2): insol |
| form | A solid |
| pka | 9.82±0.70(Predicted) |
| Stability | Light Sensitive |
| InChIKey | WKPWGQKGSOKKOO-YHBUUTGONA-N |
SAFETY
Risk and Safety Statements
| Symbol(GHS) |  GHS06 |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Signal word | Danger | |||||||||
| Hazard statements | H300+H310-H331 | |||||||||
| Precautionary statements | P261-P262-P264-P270-P271-P280-P301+P310-P321-P330-P302+P352-P304+P340-P311-P361+P364-P403+P233-P405-P501 | |||||||||
| Toxicity | LD50 in rats (mg/kg): 0.48 s.c. (Mugera, Ward) | |||||||||
| NFPA 704 |
|
Maytansine Chemical Properties,Uses,Production
Description
This novel ansa macrolide alkaloid occurs in a number of May tan us species. The structure given has been elucidated from chemical and spectroscopic data. In low doses (25-50 mcg/kg), maytansine prolonged the survival of mice bearing vincristine-sensitive P388 leukemia but not those bearing vincristine-resistant tumour lines. In vitro, it suppressed the growth of U21 0, LSl78Y and P388 leukemia cells with ED50 of 2 X 10-9 , 1.5 X 10-9 and 6 X 10-10M respectively and elevated the mitotic index in L 121 0 cells. From labelling studies it was shown that the alkaloid inhibited DNA formation by P388 cells to a greater extent than RNA and protein synthesis. Maytensine did not inhibit RNA polymerase from Escherichia coli at levels as high as 1 X 10-4M.
Uses
Antineoplastic.
Definition
ChEBI: An organic heterotetracyclic compound and 19-membered macrocyclic lactam antibiotic originally isolated from the Ethiopian shrub Maytenus serrata but also found in other Maytenus species. It exhibits cytotoxicity against many tumo r cell lines.
Biological Functions
Maytansine is a potent microtubule-targeted compound that inhibits proliferation of cells at mitosis. Antibody-maytansinoid conjugates consisting of maytansinoids (DM1 and DM4) attached to tumor-specific antibodies have shown promising clinical results The microtubule-targeting maytansinoids accumulate in cells and induce mitotic arrest at 250- to 1000-fold lower concentrations than those required for their association with tubulin or microtubules.
Toxicology
Maytansine, an ansa macrolide isolated from African plants of the genera Maytenus and Putterlickia was first described almost two decades ago. It had been reported to be active against several forms of cancer, but a later phase II evaluation suggested no major role for this drug in tumor treatment. Although the toxic side effects were moderate, the antitumor activity was also not impressive.
Maytansine binds to tubulin rapidly and reversibly. Competitive inhibition of binding between maytansine and the vinca alkaloids has been observed, suggesting that maytansine must occupy at least one of vinblastine's binding sites on the tubulin molecule. The number of the maytansine binding sites is not known. Assembly of MT is inhibited at maytansine concentrations below 1μM. This suggests a substoichiometric poisoning mechanism as in the cases of colchicine, vinblastine and podophyllotoxin, but the details are not known. In contrast to many other MT-interacting toxins,maytansine does not promote the formation of aberrant tubulin polymers, even at millimolar concentrations. In fact, low concentrations of maytansine even inhibit vinblastine-induced formation of aberrant polymers.
References
Walpert-Defillipes et al., Biochem. Pharmacol., 24, 751 (1975)
Maytansine Preparation Products And Raw materials
Raw materials
Preparation Products
Maytansine Suppliers
| Supplier | Tel | Country | ProdList | Advantage | Inquiry |
|---|---|---|---|---|---|
| CLEARSYNTH LABS LTD. | +91-22-45045900 | Hyderabad, India | 6351 | 58 | Inquiry |
| SynZeal Research Pvt Ltd | +1 226-802-2078 | Gujarat, India | 6522 | 58 | Inquiry |
| Pharmaffiliates Analytics and Synthetics P. Ltd | +91-172-5066494 | Haryana, India | 6773 | 58 | Inquiry |
| Pharma Affiliates | 172-5066494 | Haryana, India | 6761 | 58 | Inquiry |
| BOC Sciences | 16314854226; +16314854226 | United States | 19743 | 58 | Inquiry |
| ATK CHEMICAL COMPANY LIMITED | +undefined-21-51877795 | China | 32760 | 60 | Inquiry |
| TargetMol Chemicals Inc. | +1-781-999-5354 +1-00000000000 | United States | 19892 | 58 | Inquiry |
| Hubei Ipure Biology Co., Ltd | +8613367258412 | China | 10326 | 58 | Inquiry |
| Career Henan Chemica Co | +86-0371-86658258 +8613203830695 | China | 30253 | 58 | Inquiry |
| ANHUI WITOP BIOTECH CO., LTD | +8615255079626 | China | 23556 | 58 | Inquiry |
Related articles
- Maytansine: Unveiling the Potential of a Plant-Derived Compound in Targeted Cancer Therapy
- Maytansine is a potent plant-derived compound that has sparked interest in the scientific community since its discovery in 197....
- Apr 19,2024
- What is Maytansine?
- Maytansine is an important antineoplastic and antimicrobial compound with high cytotoxicity.
- Sep 7,2023
35846-53-8(Maytansine)Related Search:
1of3
chevron_right




