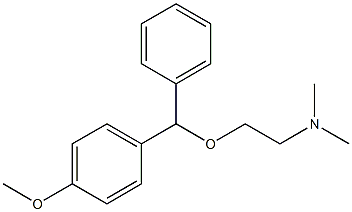
medrylamine
- CAS No.
- 524-99-2
- Chemical Name:
- medrylamine
- Synonyms
- Histaphene;medrylamine;Postafen Salve;2-(4-Methoxy-α-phenylbenzyloxy)-N,N-dimethylethanamine;2-[(4-methoxyphenyl)-phenyl-methoxy]ethyl-dimethyl-amine;2-[(4-methoxyphenyl)-phenylmethoxy]-N,N-dimethylethanamine;Ethanamine, 2-[(4-methoxyphenyl)phenylmethoxy]-N,N-dimethyl-;2-[(4-methoxyphenyl)-phenyl-methoxy]-N,N-dimethyl-ethanamine;2-((4-Methoxyphenyl)(phenyl)methoxy)-n,n-dimethylethan-1-amine
- CBNumber:
- CB8936497
- Molecular Formula:
- C18H23NO2
- Molecular Weight:
- 285.38
- MOL File:
- 524-99-2.mol
- Modify Date:
- 2023/5/4 17:34:39
SAFETY
Risk and Safety Statements
| Symbol(GHS) |  GHS07 |
|---|---|
| Signal word | Warning |
| Hazard statements | H302-H315-H319-H335 |
| Precautionary statements | P261-P305+P351+P338 |
medrylamine Chemical Properties,Uses,Production
Originator
Medrylamine,Shanghai Lansheng Corporation
Manufacturing Process
A 116 parts by weight of phenyl-p-methoxy-chloromethane (4- methoxybenzhydryl chloride) were added to 500 parts by weight of toluene and to the resulting mixture 65 parts by weight of 2-dimethylaminoethanol was added. Then the entire mixture was refluxed for two hours. The refluxed mixture was cooled and 250 parts by weight of a 10% solution of sodium hydroxide were added. This alkaline mixture was then steam distilled until the distillate was only weakly alkaline, for example a pH 7.5-8.
The residue of this steam distillation was then mixed with 200 parts by weight
of benzene and washed with water until the wash waters were practically
neutral. The benzene solution was then evaporated to dryness until the
resulting mixture was of constant weight (145 parts). This product was oily
and was then dissolved in 3000 parts by weight of dry ether; and treated
while being agitated with the theoretically equivalent amount of dry
hydrochloric acid dissolved in ether. This product is an oily product, which
solidified after standing overnight in an ice box. The ether solution was
decanted off and the solidified residue was dissolved in 1500 parts by weight
of dioxane, and then precipitated with 3000 parts by weight of ether while
being continuously agitated. The resulting solid product was the hydrochloride
of 2-(p-methoxy-α-phenylbenzyloxy)-N,N-dimethylethylamine 100 parts by
weight of the desired product were obtained corresponding to a yield of 62%
and having a melting point of 141°C.
Therapeutic Function
Antihistaminic
medrylamine Preparation Products And Raw materials
Raw materials
Preparation Products
| Supplier | Tel | Country | ProdList | Advantage | Inquiry |
|---|---|---|---|---|---|
| Jilin Chinese Academy of Sciences-yanshen Technology | +undefined18143011203 | China | 42057 | 58 | Inquiry |
| Jilin Chinese Academy of Sciences-yanshen Technology | 18143011203 | China | 41844 | 58 | Inquiry |
| Lanospharma Laboratories Co.,Ltd | 13440048448 | China | 6343 | 56 | Inquiry |
| Shanghai Kaiwei Chemical Technology Co., Ltd. | 021-58461859 15821823057 | China | 49323 | 58 | Inquiry |
| Shaanxi Dideu Medichem Co. Ltd | 029-81124267 15229202216 | China | 9998 | 58 | Inquiry |
| Aikon International Limited | 025-66113011 18112977050 | China | 15495 | 58 | Inquiry |





