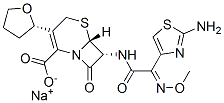
Cefovecin sodium
- CAS No.
- 141195-77-9
- Chemical Name:
- Cefovecin sodium
- Synonyms
- UK287;UK 287;D01681;C13142;074-02;UK-287;UK287,074-02;UK 287,074-02;UK-287,074-02;Cefovecin SodiuM
- CBNumber:
- CB91260675
- Molecular Formula:
- C17H18N5NaO6S2
- Molecular Weight:
- 0
- MOL File:
- 141195-77-9.mol
- MSDS File:
- SDS
- Modify Date:
- 2024/3/8 9:17:02
Cefovecin sodium Chemical Properties,Uses,Production
Description
Cefovecin sodium is a semi-synthetic broad-spectrum antibacterial agent from the cephalosporin class of chemotherapeutic agents. Cefovecin is the non-proprietary designation for (6R,7R)-7- [[(2Z)-(2-amino-4-thiazolyl)(methoxyimino)acetyl]amino]-8-oxo-3-[(2S)-tetrahydro-2-furanyl]-5-thia-1- azabicyclo[4.2.0]oct-2-ene-2-carboxylic acid, monosodium salt.
Cefovecin Sodium is an antibiotic of the cephalosporin class. It is used for the treatment of skin infections caused by Pasturella multocida in cats, and Staphylococcus intermedius/S. canis in dogs. Cefovecin Sodium is the first single-dose injectable antibiotic for dogs and cats.
Uses
Sodium salt of Cefovecin, a cephalosporin antibiotic used in the treatment of skin conditions in dogs and cats.
Indications
Dogs
Cefovecin sodium is indicated for the treatment of skin infections (secondary superficial pyoderma, abscesses,
and wounds) in dogs caused by susceptible strains of Staphylococcus intermedius and Streptococcus canis (Group G).
Cats
Cefovecin sodium is indicated for the treatment of skin infections (wounds and abscesses) in cats caused by susceptible strains of Pasteurella multocida.
Side effects
In cats, the most common effects are vomiting, diarrhea, decreased appetite, lethargy, hyperactivity or “acting strange” and inappropriate urination.
In dogs, the most common adverse effects were lethargy, decreased appetite, vomiting, diarrhea, blood in feces, dehydration, and flatulence.
Safety
Dogs
CONVENIA administered to healthy four month old dogs at doses of 12 mg/kg (1.5 X), 36 mg/kg (4.5 X), and 60 mg/kg (7.5 X) every seven days by dorsoscapular subcutaneous injections was well-tolerated for a total of 5 doses. Vomiting and diarrhea were seen in all treatment groups, with the incidence of vomiting and the incidence and duration of diarrhea increasing in a dose-related manner. Injection site irritation and transient edema occurred with increasing frequency in a dose-related manner and with repeat injections. Two injection site reactions included a seroma over the shoulder and swelling lasting > 30 days. Dogs dosed at 36 mg/kg had a significant (p = 0.0088) increase in BUN (all means remained within the normal range) compared to the controls. One dog dosed at 60 mg/kg exhibited a glomerulopathy on histopathology, and one dog in this same group had minimal peliosis hepatis. At an exaggerated dose of 180 mg/kg (22.5X) in dogs, CONVENIA caused some injection site irritation, vocalization and edema. Edema resolved within 8-24 hours.
www.zoetisus.com
Cefovecin sodium Preparation Products And Raw materials
Raw materials
Preparation Products
| Supplier | Tel | Country | ProdList | Advantage | Inquiry |
|---|---|---|---|---|---|
| CLEARSYNTH LABS LTD. | +91-22-45045900 | Hyderabad, India | 6351 | 58 | Inquiry |
| Masteam Bio-tech Co., Ltd. | +86-15200345168 +86-15200345168 | CHINA | 153 | 58 | Inquiry |
| BOC Sciences | +1-631-485-4226 | United States | 19553 | 58 | Inquiry |
| ENBRIDGE PHARMTECH CO., LTD. | +8613812269233 | China | 303 | 58 | Inquiry |
| Shanghai UCHEM Inc. | +862156762820 +86-13564624040 | China | 6931 | 58 | Inquiry |
| AFINE CHEMICALS LIMITED | +86-0571-85134551 | China | 15395 | 58 | Inquiry |
| Wuhan Fortuna Chemical Co., Ltd | +86-027-59207850 | China | 5964 | 58 | Inquiry |
| Baoji Guokang Healthchem co.,ltd | +8615604608665 15604608665 | CHINA | 9427 | 58 | Inquiry |
| Alfa Chemistry | +1-5166625404 | United States | 21317 | 58 | Inquiry |
| Hangzhou MolCore BioPharmatech Co.,Ltd. | +86-057181025280; +8617767106207 | China | 49739 | 58 | Inquiry |
Related articles
- Cefovecin sodium (veterinary medicine): Uses and side effects
- Cefovecin sodium is a broad-spectrum, third-generation cephalosporin antibiotic that effectively treats common canine and feli....
- Mar 8,2024
- Effects of Cefovecin Sodium
- Cefovecin Sodium, also known as Convenia, is an antibiotic of the cephalosporin class, licensed for the treatment of skin infe....
- Jan 19,2022





