IRONE
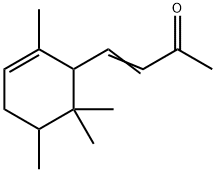
- CAS No.
- 79-69-6
- Chemical Name:
- IRONE
- Synonyms
- IRONE;α-irone;IRON-ALPHA;alpha-Irone;methyl-alpha-inon;Methyl-alpha-ionone;IRONE alpha REFINED;IRONE ALPHA 6065003;ALPHA-N-METHYLIONONE;5-methyl-alpha-ionone
- CBNumber:
- CB9184594
- Molecular Formula:
- C14H22O
- Molecular Weight:
- 206.32
- MOL File:
- 79-69-6.mol
- MSDS File:
- SDS
- Modify Date:
- 2023/5/25 18:01:22
| Melting point | <25 °C |
|---|---|
| Boiling point | 285.19°C (rough estimate) |
| Density | 0.934 g/mL at 20 °C(lit.) |
| vapor pressure | 0.4Pa at 20℃ |
| FEMA | 2597 | ALPHA-IRONE |
| refractive index |
n |
| form | Liquid |
| Odor | at 10.00 % in dipropylene glycol. orris floral berry violet woody powdery |
| Odor Type | floral |
| Merck | 13,5111 |
| JECFA Number | 403 |
| BRN | 1343498 |
| LogP | 3.8-4 at 35℃ and pH7 |
| EPA Substance Registry System | 3-Buten-2-one, 4-(2,5,6,6-tetramethyl-2-cyclohexen-1-yl)- (79-69-6) |
IRONE Chemical Properties,Uses,Production
Description
α-Irone has a characteristic orris and violet-like odor. The commercially prepared product corresponds generally to the α-isomer.
Chemical Properties
Yellowish liquid; Soluble in alcohol. A mixture of three isomers (α-, βand γ-irone).
Occurrence
Reported found in orris root, raspberry and flowers of Pittosporum sp
Uses
Perfumery, violet odor. The α isomer is also used as a flavoring agent.
Preparation
By intramolecular thermal H-ene reaction of an allysilane.
Definition
ChEBI: A methyl ketone that is alpha-ionone in which a hydrogen at position 5 of the cyclohex-2-en-1-yl ring is substituted by a methyl group.
General Description
Irone is a bioactive compound that is produced by an endophytic fungus in the rhizomes of Iris germanica.
Synthesis
Alpha-n-Methy lionone is synthesized by condensation of Methylethyl ketone with Citral, and cyclization of the resulting Pseudomethylionone. Cyclization may be carried out with Boron trifluoride or other agent.
Purification Methods
If large amounts are available, then fractionate through a Podbielniak column (p 10) or an efficient spinning band column, but small amounts are distilled using a Kügelrohr apparatus. The 4-phenylsemicarbazone has m 174-175o (165-165.5o). [IR: Seidel & Ruzicka Helv Chim Acta 35 1826 1952, Naves Helv Chim Acta 31 1280 1948, Lecomte & Naves J Chim Phys 53 462 1956, Beilstein 7 IV 378.]
IRONE Preparation Products And Raw materials
| Supplier | Tel | Country | ProdList | Advantage | Inquiry |
|---|---|---|---|---|---|
| AARAV Fragrance and Flavours Pvt. Ltd. | +912241130200 | Maharashtra, India | 12 | 58 | Inquiry |
| career henan chemical co | +86-0371-86658258 +8613203830695 | China | 29898 | 58 | Inquiry |
| Chongqing Chemdad Co., Ltd | +86-023-6139-8061 +86-86-13650506873 | China | 39916 | 58 | Inquiry |
| Shaanxi Dideu Medichem Co. Ltd | +86-29-87569266 15319487004 | China | 4088 | 58 | Inquiry |
| Zhengzhou Alfa Chemical Co.,Ltd | +8618530059196 | China | 13127 | 58 | Inquiry |
| Hebei Yanxi Chemical Co., Ltd. | +86-17531190177; +8617531190177 | China | 5959 | 58 | Inquiry |
| changzhou huayang technology co., ltd | +8615250961469 | China | 9827 | 58 | Inquiry |
| Alfa Chemistry | United States | 24072 | 58 | Inquiry | |
| TargetMol Chemicals Inc. | +1-781-999-5354 | United States | 19973 | 58 | Inquiry |
| Hebei Kingfiner Technology Development Co.Ltd | +86-15532196582 +86-15373005021 | China | 2989 | 58 | Inquiry |






