Chlorine trifluoride

- CAS No.
- 7990-91-2
- Chemical Name:
- Chlorine trifluoride
- Synonyms
- CHLORINE TRIFLUORIDE;Trifluoride chlorine;Chlorine trifluoride ISO 9001:2015 REACH
- CBNumber:
- CB92130379
- Molecular Formula:
- ClF3
- Molecular Weight:
- 92.45
- MOL File:
- 7990-91-2.mol
Chlorine trifluoride Chemical Properties,Uses,Production
Description
Chlorine trifluoride (CIF3) is a toxic, corrosive, very reactive liquefied compressed gas packaged in cylinders as a liquid under its own vapor pressure of 1.55 kg/cm2 at 21°C (22 psia at 70°F). CIF3 is a very useful chemical in operations requiring a highenergy fluorinating agent or incendiary material, especially since it can be handled at room temperatures.
Chlorine trifluoride is primarily of interest as a component in rocket fuels, in industrial cleaning and etching operations primarily in the semiconductor industry, nuclear reactor fuel processing and other industrial operations.
Chemical Properties
Chlorine trifluoride appears as a colorless gas or green liquid with a pungent odor. Boils at 53°F. It reacts with water to form chlorine and hydrofluoric acid with release of heat.
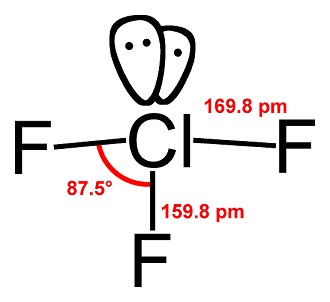
Uses
Chlorine trifluoride is used as a fluorinating agent. It may be Used as a fluorinating agent, incendiary, igniter and propellant for rockets, in nuclear reactor fuel processing, pyrolysis inhibitor for fluoro carbon polymers.
During Word War II, Chlorine trifluoride was used by Germany as an incendiary gas.
Preparation
Chlorine trifluoride was first reported by Ruff and Krug who prepared it by fluorination of chlorine, this also produced ClF and the mixture was separated by distillation.
3F2 + Cl2 → 2ClF3
Health Hazard
Chlorine trifluoride is toxic by itself and also reacts with moisture to form a variety of other toxic and corrosive materials, including hydrofluoric acid. When the product escapes into the environment, it hydrolyzes with the moisture in the air or, in the case of human contact, with the moisture in the human body. Direct contact with CIF3 vapor or liquid can result in a thermal burn in addition to the chemical burns produced by the hydrolysis products.
Chemical Reactivity
Chlorine trifluoride is hypergolic (will initiate the combustion of many materials without an ignition source) with many materials. It is extremely reactive with most inorganic and organic materials. These reactions can be very violent or in some cases explosive. Therefore, all materials that come into contact with chlorine trifluoride must be evaluated.
Chlorine trifluoride hydrolyzes rapidly with moisture to form mostly hydrogen fluoride along with hydrogen chloride, chlorine monofluoride, and a variety of oxyhalogen compounds. The oxyhalogens may include chlorine dioxide, chlorous acid, chlorine oxyfluoride and oxygen difluoride.
Chlorine trifluoride is a strong oxidizer that can essentially decrease the ignition temperature of potential fuels, including materials of construction (e.g., metals) for CIF3 systems. Furthermore, because of chlorine trifluoride’s extreme reactivity, there is a high potential for contamination to serve as an ignition source. Friction between two materials can generate fine particles (contaminants), which may ignite from the heat generated. Contaminants in chlorine trifluoride systems potentially can burn with sufficient heat to propagate the ignition to system components.
Waste Disposal
Return unused product to the supplier for proper disposal. In process applications, gaseous chlorine trifluoride can be disposed of in either liquid or dry scrubbers. Dry scrubbers work well under normal operating conditions for small quantities of Chlorine trifluoride but are not recommended for large or emergency releases unless specifically designed. Scrubbers must be designed to withstand the heat generated in the event of a large release. For normal operation, it is recommended an inert gas be used as a diluents prior to product being introduced to scrubber. This will help disperse the heat of reaction. Wet scrubbers typically use caustic solutions, such as potassium or sodium hydroxide, as the scrubbing medium. Wet scrubbers handled the heat of reaction better, as well as neutralize the products of reaction. Disposal of liquid chlorine trifluoride is extremely hazardous and is not recommended.
Chlorine trifluoride Preparation Products And Raw materials
Raw materials
Preparation Products
Chlorine trifluoride Suppliers
| Supplier | Tel | Country | ProdList | Advantage | Inquiry |
|---|---|---|---|---|---|
| Triveni chemicals | 08048762458 | New Delhi, India | 6093 | 58 | Inquiry |
| Shanghai Daken Advanced Materials Co.,Ltd | +86-371-66670886 | China | 16209 | 58 | Inquiry |
| Shaanxi Dideu Medichem Co. Ltd | +86-029-89586680 +86-18192503167 | China | 9030 | 58 | Inquiry |
| CD Chemical Group Limited | +8615986615575 | China | 20356 | 58 | Inquiry |
| Henan Fengda Chemical Co., Ltd | +86-371-86557731 +86-13613820652 | China | 20327 | 58 | Inquiry |
| Shaanxi Xihua Chemical Industry Co., Ltd | 029-81122149 13992773979 | China | 5818 | 58 | Inquiry |
| DWS Specialty Gas Co., Ltd | 159-0619-7626 13194677939 | China | 454 | 58 | Inquiry |
| Isotope (Xiamen) Industry and Trade Co., Ltd | 0510-051082803581 15903302207 | China | 229 | 58 | Inquiry |
| Shandong Hemao New Material Co., Ltd. | 13061243681 | China | 223 | 58 | Inquiry |
| chemfish tokyo co.,ltd | 0731-85567275 13517472705 | China | 1312 | 58 | Inquiry |





