Methylcyclopentadienyl manganese tricarbonyl
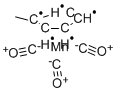
- CAS No.
- 12108-13-3
- Chemical Name:
- Methylcyclopentadienyl manganese tricarbonyl
- Synonyms
- carbon monoxide,manganese,5-methylcyclopenta-1,3-diene;CI-2;MCMT;Ak-33x;mmt[qr];ci-2[qr];EthylMMT;NSC22316;Hitec3000;ak-33x[qr]
- CBNumber:
- CB9231289
- Molecular Formula:
- C9H7MnO35*
- Molecular Weight:
- 218.09
- MOL File:
- 12108-13-3.mol
- MSDS File:
- SDS
- Modify Date:
- 2024/8/5 12:01:54
| Melting point | -1 °C (lit.) |
|---|---|
| Boiling point | 232-233 °C (lit.) |
| Density | 1.38 g/mL at 25 °C (lit.) |
| vapor density | >1 (vs air) |
| vapor pressure | 0.05 mm Hg ( 20 °C) |
| refractive index |
n |
| Flash point | 205 °F |
| storage temp. | Poison room |
| form | liquid |
| color | yellow |
| Specific Gravity | 1.388 |
| Water Solubility | Insoluble in water. Soluble in hydrocarbon solvents, alcohols, ether, acetone |
| Sensitive | Air Sensitive |
| Merck | 14,6223 |
| Exposure limits |
ACGIH: TWA 0.2 mg/m3 (Skin) OSHA: Ceiling 5 mg/m3 NIOSH: IDLH 500 mg/m3; TWA 0.2 mg/m3; TWA 1 mg/m3; STEL 3 mg/m3 |
| Stability | Stable. Incompatible with strong oxidizing agents. |
| InChIKey | LYHJNAIHGFWRKM-UHFFFAOYSA-N |
| LogP | 3.4 at 26℃ |
| CAS DataBase Reference | 12108-13-3(CAS DataBase Reference) |
| EPA Substance Registry System | Methylcyclopentadienyl manganese tricarbonyl (12108-13-3) |
SAFETY
Risk and Safety Statements
| Symbol(GHS) |    GHS06,GHS08,GHS09 |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Signal word | Danger | |||||||||
| Hazard statements | H301-H310+H330-H315-H372-H410 | |||||||||
| Precautionary statements | P262-P273-P280-P301+P310+P330-P302+P352+P310-P304+P340+P310 | |||||||||
| Hazard Codes | T,N,T+ | |||||||||
| Risk Statements | 23/24/25-40-50/53-48/23-38-26-24/25 | |||||||||
| Safety Statements | 23-26-36/37/39-45-61-60-36/37-28 | |||||||||
| RIDADR | UN 3281 6.1/PG 2 | |||||||||
| OEB | C | |||||||||
| OEL | TWA: 0.2 mg/m3 [skin] | |||||||||
| WGK Germany | 3 | |||||||||
| RTECS | OP1450000 | |||||||||
| TSCA | Yes | |||||||||
| HazardClass | 6.1 | |||||||||
| PackingGroup | II | |||||||||
| Toxicity | LD50 orally in mice, guinea pig, rabbit: 352; 905; 95 mg/kg; orally in male, female rats: 175 ±33; 89 ±14; LC50 inhalation in rat: 0.22 mg/l (Stara) | |||||||||
| NFPA 704 |
|
Methylcyclopentadienyl manganese tricarbonyl price More Price(9)
| Manufacturer | Product number | Product description | CAS number | Packaging | Price | Updated | Buy |
|---|---|---|---|---|---|---|---|
| Sigma-Aldrich | 317632 | (Methylcyclopentadienyl)manganese(I) tricarbonyl | 12108-13-3 | 1G | ₹2229.95 | 2022-06-14 | Buy |
| Sigma-Aldrich(India) | 317632 | (Methylcyclopentadienyl)manganese(I) tricarbonyl | 12108-13-3 | 1G | ₹2229.95 | 2022-06-14 | Buy |
| Sigma-Aldrich | 317632 | (Methylcyclopentadienyl)manganese(I) tricarbonyl | 12108-13-3 | 50G | ₹22505.18 | 2022-06-14 | Buy |
| Sigma-Aldrich | 317632 | (Methylcyclopentadienyl)manganese(I) tricarbonyl | 12108-13-3 | 50G | ₹22505.18 | 2022-06-14 | Buy |
| Sigma-Aldrich(India) | 317632 | (Methylcyclopentadienyl)manganese(I) tricarbonyl | 12108-13-3 | 50G | ₹22505.18 | 2022-06-14 | Buy |
Methylcyclopentadienyl manganese tricarbonyl Chemical Properties,Uses,Production
Chemical Properties
An orange liquid with a pleasant odor. Slightly soluble in water and denser than water. May be toxic by inhalation, ingestion and/or skin absorption. Faintly pleasant, herb-like odor.
Uses
Methylcyclopentadienyl manganese tricarbonyl is used for doping of ZnSe nanocrystals
It can also be used as a reactant for:
Aldol addition reactions
Preparation of homo- and heteronuclear mixed biscarbene complexes with conjugated bithiophene units
Molecular fragmentation using shaped femtosecond laser pulses
Preparation of MnAs thin films grown on GaAs(001) by metalorganic vapour phase epitaxy (MOVPE)
Preparation
Into a reaction flask under nitrogen was placed 1.26 parts bis(methylcyclopentadienyl) manganese (91.8% pure), 0.93 parts manganous acetate, 0.78 parts tetrahydrofuran (THF) and 17.31 parts toluene. Over a period of 15 minutes, a solution of 1.24 parts triethyl aluminum (TEA) in 8.70 parts of toluene was added to the above mixture with a vigorous stirring (Al/Mn atom ratio 1/1, TEA/THF mole ratio 1/1). The solution darkened slightly. This solution of the intermediate complex was transferred under nitrogen to a stainless steel autoclave. The autoclave was sealed, pressurized twice to 300 psig with carbon monoxide and vented and finally pressurized with carbon monoxide to 600 psig and heated while stirring to 100° C. Carbon monoxide was added as needed to maintain 600 psig. After two hours at 100° C., the temperature was raised to 150° C. for 30 minutes. The autoclave was then cooled, vented and discharged. The mixture was hydrolyzed with 10% aqueous HCl. An equal volume of pentane was added to extract the MMT. The pentane phase was analyzed by gas chromoatograph (GC) using a pentadecane internal standard to show a yield of Methylcyclopentadienyl manganese tricarbonyl(MMT) based on manganese of 84% and, based on MCP, of 89%.
Definition
Methylcyclopentadienyl Manganese Tricarbonyl is a dark orange liquid with a faintly pleasant odor. It is used as a fuel additive to abate smoke and as a gasolinc additive in antiknock mixes.
General Description
Methylcyclopentadienyl manganese tricarbonyl (hereinafter "MMT") is an antiknock agent for gasoline discovered in the fifties and sold commercially by Ethyl Corporation. It can be made by the reaction of carbon monoxide with bis(methylcyclopentadienyl) manganese referred to as "carbonylation".
Air & Water Reactions
Slightly soluble in water.
Reactivity Profile
METHYLCYCLOPENTADIENYLMANGANESE TRICARBONYL decomposes when exposed to light. METHYLCYCLOPENTADIENYLMANGANESE TRICARBONYL is stable in water.
Hazard
Toxic by ingestion, inhalation, and skinabsorption. Central nervous system impairment;lung, liver and kidney damage.
Health Hazard
In concentrated form METHYLCYCLOPENTADIENYLMANGANESE TRICARBONYL is highly toxic by all routes of exposure. Approximately 5-15 ml, when spilled on the hand and wrist of a worker, produced toxic effects within 3-5 minutes.
Fire Hazard
When heated to decomposition, METHYLCYCLOPENTADIENYLMANGANESE TRICARBONYL emits toxic fumes of carbon monoxide. Hazardous polymerization may not occur.
Safety Profile
Poison by ingestion, inhalation, skin contact, intravenous, and intraperitoneal routes. A slimn irritant. When heated to decomposition it emits toxic fumes of CO. See also MANGANESE COMPOUNDS and CARBONYLS.
Potential Exposure
MMT is used as an octane improver in unleaded gasoline, other distillate fuels, and fuel oils; as a smoke abater in fuels.
Shipping
UN3281 Metal carbonyls, liquid n.o.s. Hazard class 6.1. Technical name required, Potential Inhalation Hazard (Special Provision 5).
Incompatibilities
Light causes decomposition. May be air-reactive. A strong reducing agent. Incompatible with oxidizers (chlorates, nitrates, peroxides, permanganates, perchlorates, chlorine, bromine, fluorine, etc.); contact may cause fires or explosions. Keep away from alkaline materials, strong bases, strong acids, oxoacids, epoxides, halogens.
Waste Disposal
Generators of waste (equal to or greater than 100 kg/mo) containing this contaminant, EPA hazardous waste number N450, must conform to USEPA regulations for storage, transportation, treatment, and disposal of waste. Dispose of waste material as hazardous waste using a licensed disposal contractor to an approved landfill. Dispose of contents and container to an approved waste disposal plant. Containers must be disposed of properly by following package label directions or by contacting your local or federal environmental control agency, or by contacting your regional EPA office. All federal, state, and local environmental regulations must be observed. Do not discharge into drains or sewers.
Methylcyclopentadienyl manganese tricarbonyl Preparation Products And Raw materials
| Supplier | Tel | Country | ProdList | Advantage | Inquiry |
|---|---|---|---|---|---|
| CHEMSWORTH | +91-261-2397244 | New Delhi, India | 6707 | 30 | Inquiry |
| Alfa Aesar | 1 800 209 7001 | Maharashtra, India | 6913 | 58 | Inquiry |
| SIMAGCHEM CORP | +86-13806087780 | China | 17367 | 58 | Inquiry |
| Tianjin Zhongxin Chemtech Co., Ltd. | +86-022-66880623 +8618622897568 | China | 562 | 58 | Inquiry |
| Xiamen AmoyChem Co., Ltd | +86-592-6051114 +8618959220845 | China | 6387 | 58 | Inquiry |
| Jinan Qinmu Fine Chemical Co.,Ltd. | +8618660799346 | China | 1009 | 58 | Inquiry |
| Hebei Guanlang Biotechnology Co., Ltd. | +86-19930503282 | China | 8822 | 58 | Inquiry |
| Hebei Guanlang Biotechnology Co., Ltd. | +8619930503259 | China | 18433 | 58 | Inquiry |
| Hebei Yanxi Chemical Co., Ltd. | +86-17531190177; +8617531190177 | China | 5873 | 58 | Inquiry |
| airuikechemical co., ltd. | +undefined86-15315557071 | China | 983 | 58 | Inquiry |
Related articles
- Methylcyclopentadienyl Manganese Tricarbonyl: Plant Uptake, Effects on Metabolism and Neurotoxic Effects
- Methylcyclopentadienyl manganese tricarbonyl in gasoline leads to plant manganese uptake, affecting metabolism and causing neu....
- Feb 23,2024
- Methylcyclopentadienyl manganese tricarbonyl: properties, applications and environmental hazards
- Methylcyclopentadienyl Manganese Tricarbonyl is a fuel additive enhancing combustion efficiency and reducing emissions, yet co....
- Nov 29,2023
- What are the uses and toxicity of Methylcyclopentadienyl manganese tricarbonyl
- MMT is used as an antiknock agent and octane promoter in unleaded gasoline, but is highly toxic.
- Sep 4,2023





